- #1
lavoisier
- 177
- 24
Hi everyone,
I've recently had a little discussion at work concerning the concept of aromaticity applied to some molecules.
To cut a long story short, while I see why 2-pyridone is considered aromatic:
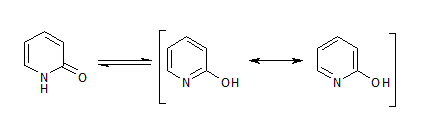
I don't understand why 1-methyl-2-pyridone is also considered aromatic:
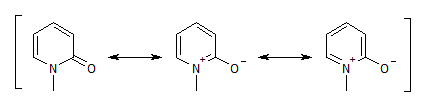
The two structures on the right do respect the rules for aromaticity (6 pi electrons, cyclic delocalisation, etc).
However, if a molecule must be seen as the average of its resonance structures, 'weighted' on their relative stability, then a molecule whose only aromatic resonance structures are very unstable (e.g. by charge separation as above), will be represented more closely by non-aromatic structures, and on the whole it won't 'be' aromatic. Or am I wrong?
I had a look at this nice review:
http://www.ark.chem.ufl.edu/published_papers/pdf/1074.pdf
which however didn't clarify the situation in my mind.
To add to the confusion, in the past I did a chlorination of a fused pyridine-2,4-dione, which gave this:
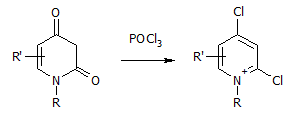
We expected the chlorination to go only once on either carbonyl, yielding 2 isomeric mono-Cl products, and instead we got the dichloro compound with a positive charge. The justification was that the latter was more stable than expected due its aromatic character. It was so stable that it took a rather long time to hydrolyse off one Cl at pH 10 (large excess of aqueous carbonate). And we could even isolate it as a solid salt.
This fact is used as an argument to say that charged structures are perfectly valid.
But for me this is a different case, as here there is no *separation* of charge, and all resonance structures have one positive charge on one atom, there is no possible structure with no charges anywhere.
So... not clear at all, as far as I'm concerned.
I would like to know your opinion on the subject, please.
Thanks!
L
I've recently had a little discussion at work concerning the concept of aromaticity applied to some molecules.
To cut a long story short, while I see why 2-pyridone is considered aromatic:
I don't understand why 1-methyl-2-pyridone is also considered aromatic:
The two structures on the right do respect the rules for aromaticity (6 pi electrons, cyclic delocalisation, etc).
However, if a molecule must be seen as the average of its resonance structures, 'weighted' on their relative stability, then a molecule whose only aromatic resonance structures are very unstable (e.g. by charge separation as above), will be represented more closely by non-aromatic structures, and on the whole it won't 'be' aromatic. Or am I wrong?
I had a look at this nice review:
http://www.ark.chem.ufl.edu/published_papers/pdf/1074.pdf
which however didn't clarify the situation in my mind.
To add to the confusion, in the past I did a chlorination of a fused pyridine-2,4-dione, which gave this:
We expected the chlorination to go only once on either carbonyl, yielding 2 isomeric mono-Cl products, and instead we got the dichloro compound with a positive charge. The justification was that the latter was more stable than expected due its aromatic character. It was so stable that it took a rather long time to hydrolyse off one Cl at pH 10 (large excess of aqueous carbonate). And we could even isolate it as a solid salt.
This fact is used as an argument to say that charged structures are perfectly valid.
But for me this is a different case, as here there is no *separation* of charge, and all resonance structures have one positive charge on one atom, there is no possible structure with no charges anywhere.
So... not clear at all, as far as I'm concerned.
I would like to know your opinion on the subject, please.
Thanks!
L
Last edited by a moderator: