greg_rack
Gold Member
- 361
- 79
- Homework Statement
- The radioactive isotope X becomes the stable isotope Y after a succession of decays involving only the emission of alpha and beta (β–) particles.
During the decay of one nucleus from X to Y, a total of seven particles are emitted. It is known that more of these particles are alpha particles than beta particles.
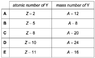
The atomic number of X is Z and the mass number of X is A.
Which row in the table(attached to the "solution" section) could give the atomic number and the mass number of Y?
- Relevant Equations
- None
Last edited by a moderator: