Discussion Overview
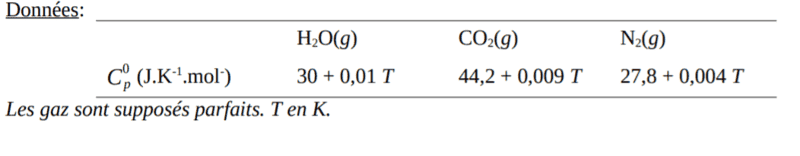
The discussion revolves around calculating the maximum flame temperature during the combustion of methane (CH4). Participants are exploring the theoretical aspects of combustion temperatures, including the impact of different reactant compositions and the use of heat capacity values in calculations.
Discussion Character
- Technical explanation
- Debate/contested
- Mathematical reasoning
Main Points Raised
- One participant states they calculated a maximum flame temperature of 4805 K but could not replicate this result and seeks assistance in identifying their error.
- Another participant questions the composition of visible flames and its relevance to the discussion.
- A participant points out a mathematical correction regarding the formula used for temperature calculation, suggesting that the expression should be (Tf2 - 2982) instead of (Tf - 298)2, which they claim leads to the 4805 K result.
- There is skepticism about the correctness of the 4805 K answer, with a participant questioning the necessity of including the heat capacity of nitrogen (N2) in the calculations.
- A participant clarifies that the heat capacity of N2 was provided for a different combustion scenario involving a mixture of 20% O2 and 80% N2, while the current discussion focuses on combustion in 100% O2.
- One participant mentions obtaining a temperature of 2280 K for the combustion in 20% O2 and 80% N2 and asks if this result is correct.
Areas of Agreement / Disagreement
Participants express differing views on the correctness of the calculated maximum flame temperature and the relevance of certain parameters in the calculations. The discussion remains unresolved regarding the accuracy of the temperature values and the implications of using different reactant compositions.
Contextual Notes
There are limitations regarding the assumptions made in the calculations, particularly concerning the heat capacities used and the specific conditions of the combustion reactions being analyzed.