Janak Preet
- 2
- 0
Hello!
I've been working on a few soil based MFCs and I have been monitoring my voltage and energy output for a week now. I've made the electrodes by covering stainless steel metal meshes with activated carbon using epoxy. I've gotten some funky results as attached.
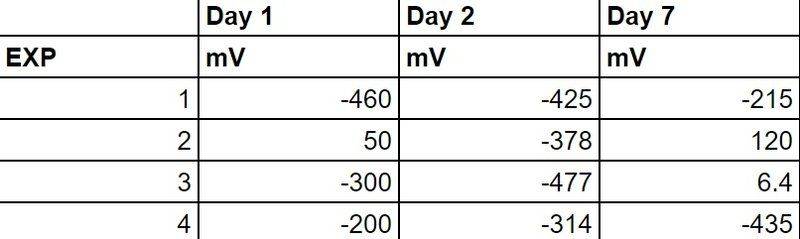
As you can see... the voltage provided a negative value (and an anomalous positive value for exp 2 on day 1), then after waiting a few more days the results show positive for exp 2 and exp 3.
I have a theory that the voltage I have been generating was not due to microbial action, but rather due to galvanic corrosion of the stainless steel, with the following reactions:
anode (MFC cathode): M -> M+ + e-
cathode (our anode): 2H+ + 2e- -> H2 OR O2(g) + 2H2O(l) + 4e- -> 4OH-(aq).
I am unable to prove this as I can't clearly see corrosion on my top electrode... (perhaps due to it being covered in carbon powder) I do however see water droplet formation which is desired as that indicates my microbes are indeed producing electrons which are being transferred to create H2O:
MFC electrode reactions:
anode: C6H12O6 + 6 H2O → 6 CO2 + 24 H+ + 24e-
cathode: 24 H+ + 24 e- + 6 O2 → 12 H2O
Any thoughts? Does my theory make sense or is there another explanation for the change in current direction? Thanks!
I've been working on a few soil based MFCs and I have been monitoring my voltage and energy output for a week now. I've made the electrodes by covering stainless steel metal meshes with activated carbon using epoxy. I've gotten some funky results as attached.
As you can see... the voltage provided a negative value (and an anomalous positive value for exp 2 on day 1), then after waiting a few more days the results show positive for exp 2 and exp 3.
I have a theory that the voltage I have been generating was not due to microbial action, but rather due to galvanic corrosion of the stainless steel, with the following reactions:
anode (MFC cathode): M -> M+ + e-
cathode (our anode): 2H+ + 2e- -> H2 OR O2(g) + 2H2O(l) + 4e- -> 4OH-(aq).
I am unable to prove this as I can't clearly see corrosion on my top electrode... (perhaps due to it being covered in carbon powder) I do however see water droplet formation which is desired as that indicates my microbes are indeed producing electrons which are being transferred to create H2O:
MFC electrode reactions:
anode: C6H12O6 + 6 H2O → 6 CO2 + 24 H+ + 24e-
cathode: 24 H+ + 24 e- + 6 O2 → 12 H2O
Any thoughts? Does my theory make sense or is there another explanation for the change in current direction? Thanks!