- #1
mjmnr3
- 5
- 0
- Homework Statement
- Consider now a particle, which can sit in two harmonic oscillators. Oscillator A has
energy levels ε=nhω, and oscillator B has energy levels ε= ε_0 + nhω, where ε_0 > 0 and
n = 0, 1, 2, . . .. The particle can hop freely between the two oscillators, and the available
states for the particle are therefore the harmonic oscillator levels in A and B.
Question b:
Give an expression for the partition function for the particle, when the temperature is T.
What is the probability P that the particle sits in oscillator A?
- Relevant Equations
- $$
z=\sum_{s} e^{-E(s) / k T}
$$
Here is the solution I have been given:
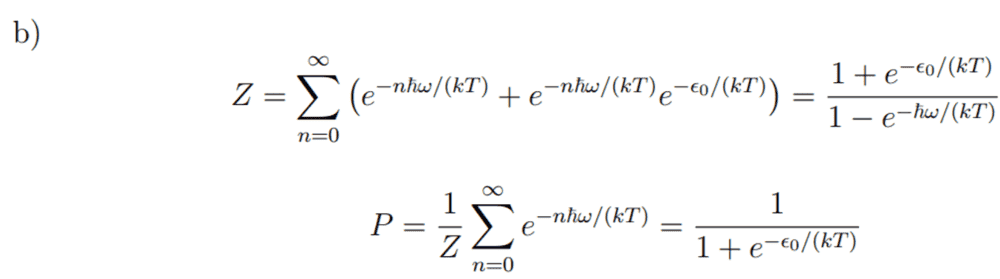
But I really don't understand this solution. Why can I just add these two exponential factors (adding two individual partition functions?).-------------------------------------------------------------------------------------------------------------------------------------
if ##E_{A}=n \hbar \omega## and ##E_{B}=n \hbar \omega+\epsilon_{0},## Why is
$$
z=\sum_{n=0}^{\infty} e^{-E_{A} / k T}+\sum_{n=0}^{\infty} e^{-E_{B} / K T}=Z_{A}+Z_{B}
$$
Because I would solve it differently. The way Daniel Schroeder (Introduction to thermal physics) teaches you to calculate the partition function is:
##z=\sum_{s}^{\infty} e^{-E(s) / k T}##, where ##s## is the different quantum states of the system. So how does the two exponential factors appear?
I interpret the system's hamiltonian consisting of a sum of the two harmonic oscillators $$\mathrm{H}=\mathrm{H}_{\mathrm{A}}+\mathrm{H}_{\mathrm{B}}$$, So what I should really find is the eigenvalues to this operator:
$$\hat{H}|s\rangle=E(s)|s\rangle$$
if ##H_{A}## and ##H_{B}## commutes, then they should have simultaneous eigenfunctions and we should expect the energy to be the sum of each hamiltons eigenvalue: ##E(s)= E_{A}+E_{B}##
So in that case, the partition function would be:
$$
z=\sum_{n=0}^{\infty} e^{-\left(E_{A}+E_{B}\right) / \kappa T}=\sum_{n=0}^{\infty} e^{-E_{A} / k T} e^{-E_{B / K T}}=\sum_{n=0}^{\infty}\left(e^{-n \hbar \omega /(k T)} e^{-n \hbar \omega /(k T)} e^{-\epsilon_{0} /(k T)}\right)
$$
So the issue, is probably that I really don't understand the setup of this system, and what they mean by one particle and two harmonic oscillators.
But I really don't understand this solution. Why can I just add these two exponential factors (adding two individual partition functions?).-------------------------------------------------------------------------------------------------------------------------------------
if ##E_{A}=n \hbar \omega## and ##E_{B}=n \hbar \omega+\epsilon_{0},## Why is
$$
z=\sum_{n=0}^{\infty} e^{-E_{A} / k T}+\sum_{n=0}^{\infty} e^{-E_{B} / K T}=Z_{A}+Z_{B}
$$
Because I would solve it differently. The way Daniel Schroeder (Introduction to thermal physics) teaches you to calculate the partition function is:
##z=\sum_{s}^{\infty} e^{-E(s) / k T}##, where ##s## is the different quantum states of the system. So how does the two exponential factors appear?
I interpret the system's hamiltonian consisting of a sum of the two harmonic oscillators $$\mathrm{H}=\mathrm{H}_{\mathrm{A}}+\mathrm{H}_{\mathrm{B}}$$, So what I should really find is the eigenvalues to this operator:
$$\hat{H}|s\rangle=E(s)|s\rangle$$
if ##H_{A}## and ##H_{B}## commutes, then they should have simultaneous eigenfunctions and we should expect the energy to be the sum of each hamiltons eigenvalue: ##E(s)= E_{A}+E_{B}##
So in that case, the partition function would be:
$$
z=\sum_{n=0}^{\infty} e^{-\left(E_{A}+E_{B}\right) / \kappa T}=\sum_{n=0}^{\infty} e^{-E_{A} / k T} e^{-E_{B / K T}}=\sum_{n=0}^{\infty}\left(e^{-n \hbar \omega /(k T)} e^{-n \hbar \omega /(k T)} e^{-\epsilon_{0} /(k T)}\right)
$$
So the issue, is probably that I really don't understand the setup of this system, and what they mean by one particle and two harmonic oscillators.