kaare_t
- 4
- 11
- TL;DR
- Approaching absolute vacuum versus not absolute vacuum with the same delta P, forces involved
Hi,
New member here. I have no higher education, please excuse me if I'm asking an obvious question, and feel free to reply with more questions if my post is unclear! And please feel free to correct me if I'm using wrong term(s), or wrong assumptions.
My question is related to pressure, and more specifically if approaching an absolute vaccum (I know it's impossible to reach 0.0 bar, but my post is more of a thought-experiment).
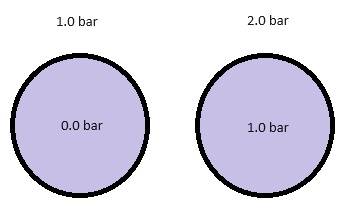
My question is if there is any difference between two configurations where the delta pressure is 1.0 bar in both configurations (see picture). The picture is of two different cylinders that are closed, and in both examples my intention is to have a delta P of 1.0 bar. Are there any other forces than the delta pressure involved in such an example, or can I assume that the only force applied is the delta P?
My own wonderings are about approaching 0.0 bar, will the forces acting on the pipe-wall look similar to e.g. an object approaching the speed of light (a curve of applied energy that exponentially grows into infinity)? And if so, can I assume that pressure is related to energy in a sense that you would need infinite energy to create an absolute vacuum?
New member here. I have no higher education, please excuse me if I'm asking an obvious question, and feel free to reply with more questions if my post is unclear! And please feel free to correct me if I'm using wrong term(s), or wrong assumptions.
My question is related to pressure, and more specifically if approaching an absolute vaccum (I know it's impossible to reach 0.0 bar, but my post is more of a thought-experiment).
My question is if there is any difference between two configurations where the delta pressure is 1.0 bar in both configurations (see picture). The picture is of two different cylinders that are closed, and in both examples my intention is to have a delta P of 1.0 bar. Are there any other forces than the delta pressure involved in such an example, or can I assume that the only force applied is the delta P?
My own wonderings are about approaching 0.0 bar, will the forces acting on the pipe-wall look similar to e.g. an object approaching the speed of light (a curve of applied energy that exponentially grows into infinity)? And if so, can I assume that pressure is related to energy in a sense that you would need infinite energy to create an absolute vacuum?
