Stormer
- 121
- 22
Hi. How do i calculate the dynamic combustion pressure, force and piston velocity for different fuels in a cylinder? Assuming perfect combustion and no thermal loss trough the walls and piston.
I am thinking about an application similar to for example a fuel driven nail gun that runs on a butane gas and air mixture.
The known parameters is:
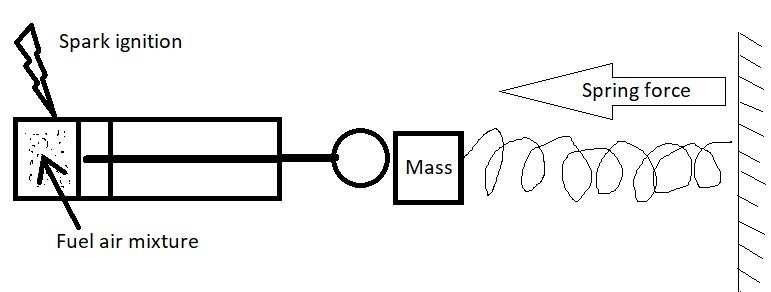
I am thinking about an application similar to for example a fuel driven nail gun that runs on a butane gas and air mixture.
The known parameters is:
- The mass the cylinder will move (M)
- A spring constant and the distance the cylinder will move (F = kx)
- The diameter of the cylinder (D)
- The initial volume and pressure of fuel air mixture (in perfect ratio).
- The fuel used (and that will give the combustion energy and therefore temperature and pressure rise in the cylinder)
Last edited: