JHUK
- 5
- 0
I posted in picture format to post on another website, but haven't found a reply yet:
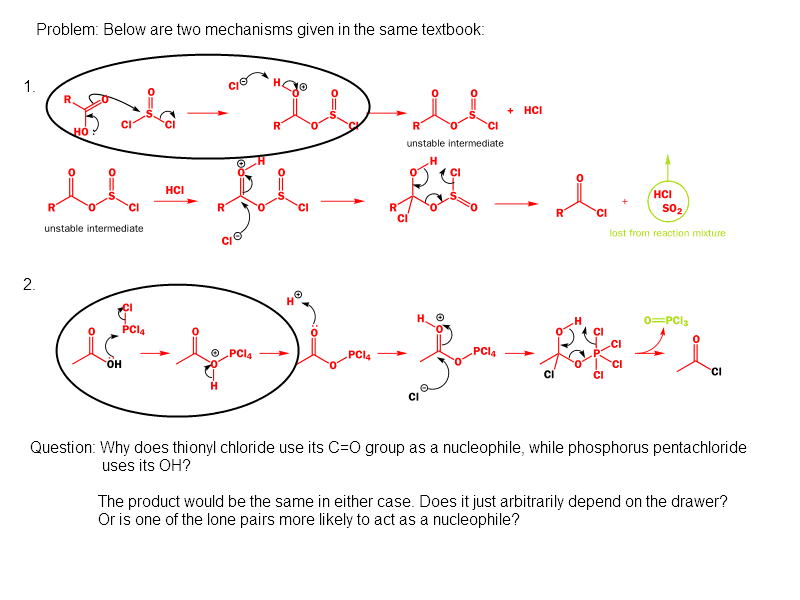
This discussion centers on the nucleophilic behavior of oxygen in carboxylic acid derivatives, specifically the -COOH group. Participants clarify that the oxygens in these groups are equivalent and can be represented by resonance structures, where hydroxyl and carbonyl oxygens interchange. The correct mechanism for nucleophilic attack involves the protonated carbonyl, while the distinction between the carboxylate anion and its protonated form is emphasized. The rapid nature of proton transfer is noted, suggesting that the mechanisms are closely related.
PREREQUISITESChemistry students, organic chemists, and researchers interested in reaction mechanisms and nucleophilic behavior in organic compounds.
Yanick said:You're question is a little unclear because the mechanism shows nucleophilic attack by a -COOH group. The oxygens in these groups are actually equivalent and are best represented by resonance structures where the hydroxyl and carbonyl oxygens exchange.
sjb-2812 said:No, they are equivalent in the carboxylate anion, but not in the protonated version. Resonance structures do not involve the movement of protons.