SchroedingersLion
- 211
- 56
Greetings!
Can you help me understand what this text book problem asks of me?
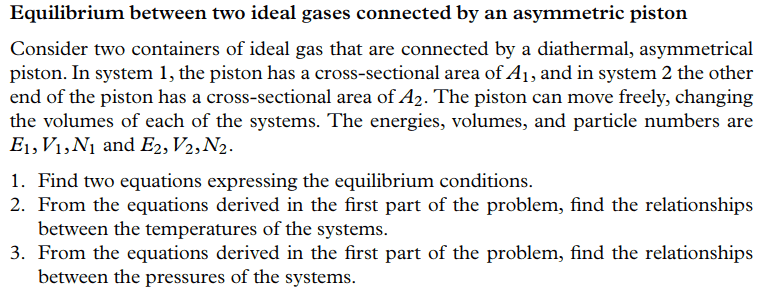
The situation was considered in the text: In equilibrium, the forces on both sides of the piston are equal: ##A_1P_1 = A_2P_2##.
This is the first equation. It should also answer part 3 of the question. The piston allows heat exchange, so that in equilibrium I have ##T_1=T_2##. This should answer part 2. To get a second equation to answer part one, I can just apply the ideal gas law to the first equation to get
$$
A_j\frac{N_j}{V_j} = A_k\frac{N_k}{V_k}.
$$
I feel like this is way too easy and I am missing something... I don't know what the author wants me to do. I should note that he derived the entropy function S(E,V,N) of the ideal gas, and also the three partial derivatives such as ##\left(\frac{\partial S}{\partial V}\right)_{E,N}=\frac {P} {T} ##, so maybe he expects some more wizardry with them?SL
Can you help me understand what this text book problem asks of me?
The situation was considered in the text: In equilibrium, the forces on both sides of the piston are equal: ##A_1P_1 = A_2P_2##.
This is the first equation. It should also answer part 3 of the question. The piston allows heat exchange, so that in equilibrium I have ##T_1=T_2##. This should answer part 2. To get a second equation to answer part one, I can just apply the ideal gas law to the first equation to get
$$
A_j\frac{N_j}{V_j} = A_k\frac{N_k}{V_k}.
$$
I feel like this is way too easy and I am missing something... I don't know what the author wants me to do. I should note that he derived the entropy function S(E,V,N) of the ideal gas, and also the three partial derivatives such as ##\left(\frac{\partial S}{\partial V}\right)_{E,N}=\frac {P} {T} ##, so maybe he expects some more wizardry with them?SL