phoenixXL
- 49
- 4
Question
Find out the total number of possible isomers with molecular formula C6H12 that contain a cyclobutane ring.
Attempted Solution
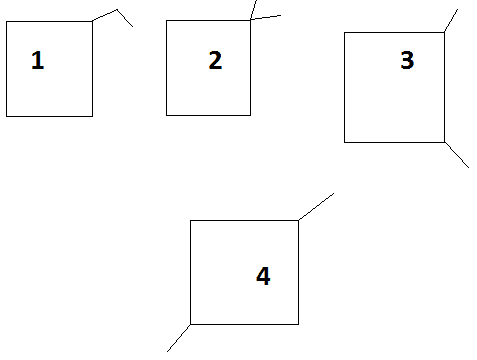
In Compound 3, Optical Isomerism is possible (geometrical isomerism is also possible but is neglected due to presence of optical isomerism) about both the branches, hence it will be counted as 4 isomers
In Compound 4, Geometrical Isomerism is possible about the two branches, therefore it will be counted as 2 isomers
Counting All : 1 + 1 + 4 + 2 = 8
Hence there are 8 isomers possible
Problem
The book where I found this question says that it consists only of 7 isomers.
Please help me out. Thanks for your time.
Find out the total number of possible isomers with molecular formula C6H12 that contain a cyclobutane ring.
Attempted Solution
In Compound 3, Optical Isomerism is possible (geometrical isomerism is also possible but is neglected due to presence of optical isomerism) about both the branches, hence it will be counted as 4 isomers
In Compound 4, Geometrical Isomerism is possible about the two branches, therefore it will be counted as 2 isomers
Counting All : 1 + 1 + 4 + 2 = 8
Hence there are 8 isomers possible
Problem
The book where I found this question says that it consists only of 7 isomers.
Please help me out. Thanks for your time.