roam
- 1,265
- 12
I heated two different materials with a laser beam for about 10 seconds and these are the measured temperature profiles:
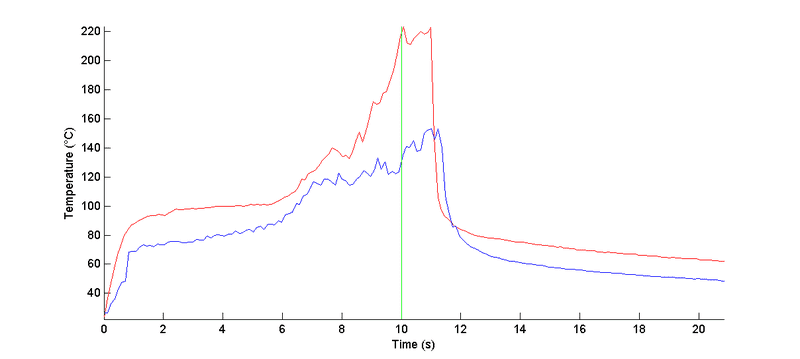
From the various solutions to the general heat conduction equation, temperature rise seems to increase with increasing thermal resistance of the material. The total thermal resistance of a material of length ##L## (according to Carslaw & Jaeger) is:
$$\overline{R}=\intop_{0}^{L}\frac{dz}{K},$$
where ##K## is the thermal conductivity of the substance, which is itself confounded with density and heat capacity.
So, is it possible to argue that the sample with the red curve has a higher thermal resistance? If so, can we also say that it has a lower density and/or heat capacity?
Any explanation is greatly appreciated.
P. S. One of the features I observed is that the temperature did not decline immediately after the laser was turned off, but rather about a second later. Is this normal? (The data is extracted from a thermographic video so there could be some errors involved)
From the various solutions to the general heat conduction equation, temperature rise seems to increase with increasing thermal resistance of the material. The total thermal resistance of a material of length ##L## (according to Carslaw & Jaeger) is:
$$\overline{R}=\intop_{0}^{L}\frac{dz}{K},$$
where ##K## is the thermal conductivity of the substance, which is itself confounded with density and heat capacity.
So, is it possible to argue that the sample with the red curve has a higher thermal resistance? If so, can we also say that it has a lower density and/or heat capacity?
Any explanation is greatly appreciated.
P. S. One of the features I observed is that the temperature did not decline immediately after the laser was turned off, but rather about a second later. Is this normal? (The data is extracted from a thermographic video so there could be some errors involved)