TomP86
- 2
- 2
Hi all. My question is regarding the mixing of 2 pigments (such as paints) to form a uniform colour in the retina.
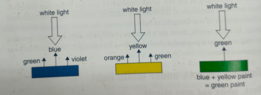
I understand mixing yellow and red create orange. This can be easy seen as red pigment will reflect a little orange light and yellow pigment will reflect a little orange light indevidually (where they are not mixed), so during the subtraction of mixing them together, the orange is the colour in common they both reflect so the eye precieved it as orange.
Simmilar, mixing yellow and blue yeilds the same result making it green with in the wave length inbetween yellow and blue.
What I am having some difficulty with is the mixing of red (780nm) with blue(450nm). There is no common reflected colour as scene in the 2 examples above (Y and O, and B and Y). Im trying to understand how our mind precieves this colour as purple.
From online searching it seems to be a biological answer based on the RGB photoreceptor cones in our eyes.
If I followed the proceedure above for calculating a mixture of red and blue it would result in black as there is no common reflected colour. But this is clearly not the case.
So when you mix 2 pigments together, what actually happens. Do the molecule from the first pigment mix together (at seeminly random) with the second pigments molecues and as the light wave reflects from each individule (separate pigment molecule) it reflects the light that that particular pigment molocule does not obsorb?
or is there a chemical reaction that bonds the 2 pigments? (My chestry knolwege is limited at the moment). And if so, what wavelength will the bonded molecule reflect?
If the answer is the former, (the surface with the mixed pigment reflects a proportion of wave pigments 1's reflecetive wavelength and of pigments 2's reflective wavelength depending on the ratio quantity that was mixed), then the perception of the purple is a color calculated by the human mind? Or is there a physical explaination?
Thank you very much in advance,
Yours Tom P.
I understand mixing yellow and red create orange. This can be easy seen as red pigment will reflect a little orange light and yellow pigment will reflect a little orange light indevidually (where they are not mixed), so during the subtraction of mixing them together, the orange is the colour in common they both reflect so the eye precieved it as orange.
Simmilar, mixing yellow and blue yeilds the same result making it green with in the wave length inbetween yellow and blue.
What I am having some difficulty with is the mixing of red (780nm) with blue(450nm). There is no common reflected colour as scene in the 2 examples above (Y and O, and B and Y). Im trying to understand how our mind precieves this colour as purple.
From online searching it seems to be a biological answer based on the RGB photoreceptor cones in our eyes.
If I followed the proceedure above for calculating a mixture of red and blue it would result in black as there is no common reflected colour. But this is clearly not the case.
So when you mix 2 pigments together, what actually happens. Do the molecule from the first pigment mix together (at seeminly random) with the second pigments molecues and as the light wave reflects from each individule (separate pigment molecule) it reflects the light that that particular pigment molocule does not obsorb?
or is there a chemical reaction that bonds the 2 pigments? (My chestry knolwege is limited at the moment). And if so, what wavelength will the bonded molecule reflect?
If the answer is the former, (the surface with the mixed pigment reflects a proportion of wave pigments 1's reflecetive wavelength and of pigments 2's reflective wavelength depending on the ratio quantity that was mixed), then the perception of the purple is a color calculated by the human mind? Or is there a physical explaination?
Thank you very much in advance,
Yours Tom P.
Attachments
Last edited: