Tapehead2
- 16
- 0
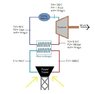
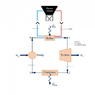
I have been looking everywhere for some equation that relates temperature and pressure at a constant volume for water. I am doing a project where I am designing a solar collector and it's thermodynamic processes and I want to ensure my values are accurate in a controlled volume. Attached is the diagram of my project thus far. Any help with regards to a confirmation of the accuracy of these values or an equation to find more accurate ones would be appreciated. Thanks!
NOTE: These values are ones I made up, my goal is to find better more reasonable values.

NOTE: These values are ones I made up, my goal is to find better more reasonable values.
Attachments
Last edited: