Erwin123
- 2
- 0
So I'm designing a proces where carbondioxide is converted into methane using the Sabatier reaction. For this reaction hydrogen is required which I'm planning on producing using the electrolysis of water. But I'm having a problem where I need the speed of this reaction to determine the size of the reactor needed. Does anyone know how I can calculate this reaction speed without using experiments? Because the normal equation for reaction rate gives me a value with a reaction rate constant which is obtained experimentally (see the figure on the right for my derived equation for the reaction rate).
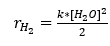
I hope I can get an answer soon
I hope I can get an answer soon
Last edited: