Discussion Overview
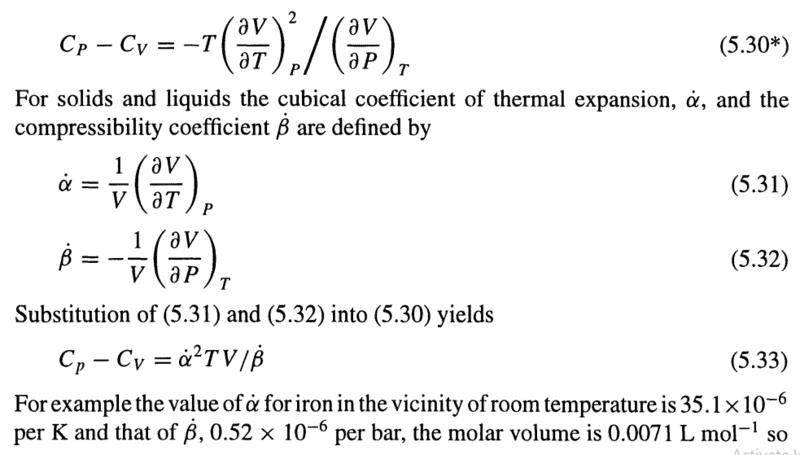
The discussion revolves around the definition and implications of thermodynamic constants, specifically the coefficients of volumetric thermal expansion (##\dot{\alpha}##) and bulk compliance (##\dot{\beta}##). Participants explore the reasoning behind including volume (V) in these definitions and the impact on understanding and measurement in thermodynamics.
Discussion Character
- Exploratory
- Debate/contested
- Technical explanation
Main Points Raised
- One participant questions the necessity of defining ##\dot{\alpha}## and ##\dot{\beta}## with respect to volume, seeking clarity on its purpose.
- Another participant explains that dividing by V simplifies the physical dimensions of ##\dot{\alpha}## and ##\dot{\beta}##, making them independent of the system's volume.
- It is noted that ##\dot{\alpha}## and ##\dot{\beta}## are not constants and vary with temperature and pressure, which is a point of contention in the discussion.
- A participant suggests that omitting V could make the coefficients more intuitive, proposing that expressing alpha in terms of volume change per temperature change might be more straightforward.
- Another participant counters that the proposed method would depend on the initial volume, while the conventional definition does not, arguing for the robustness of the established definitions.
- Further elaboration is provided on the mathematical implications of the definition of ##\dot{\alpha}##, including its integration and application in expressing thermal expansion.
- A later reply expresses appreciation for the mathematical explanation, indicating it deepened their understanding.
Areas of Agreement / Disagreement
Participants express differing views on the necessity and utility of the volume-dependent definitions of ##\dot{\alpha}## and ##\dot{\beta}##. There is no consensus on whether omitting V would enhance understanding or if the conventional definitions are superior.
Contextual Notes
Some participants highlight the variations of ##\dot{\alpha}## and ##\dot{\beta}## with temperature and pressure, suggesting that the discussion is limited by these dependencies and the definitions used.