Jaydude
- 3
- 0
I'm unsure of part b of the question 7 below;

My attempt:
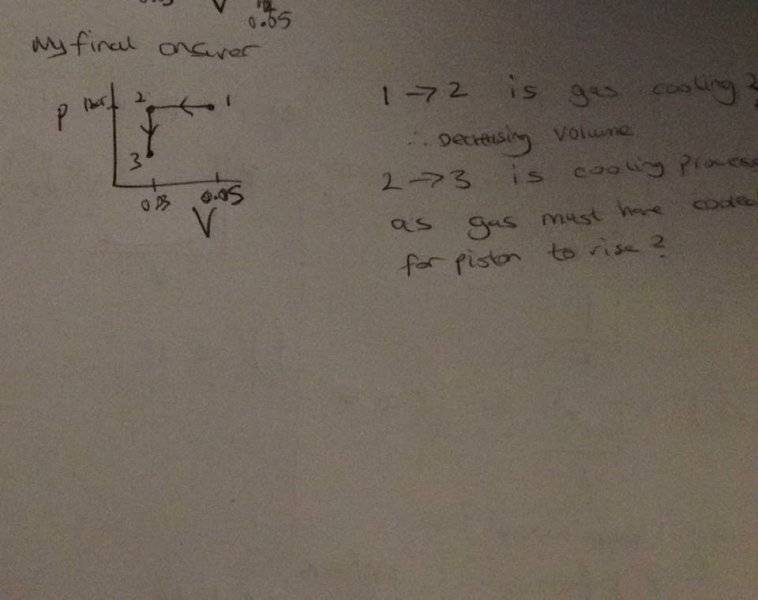
I'm confused as to what direction the arrows are meant to go.
If heat transfer occurs to the gas, and the piston rises, the volume must have decreased in the cylinder.
So from state 1 - 2, the gas is cooling or heating up? This is a isobaric process right?
And from states 2- 3 the piston moves up, known as isochoric?
Finally therefore is my process path correct?
Thanks, kind regards,
Jay
My attempt:
I'm confused as to what direction the arrows are meant to go.
If heat transfer occurs to the gas, and the piston rises, the volume must have decreased in the cylinder.
So from state 1 - 2, the gas is cooling or heating up? This is a isobaric process right?
And from states 2- 3 the piston moves up, known as isochoric?
Finally therefore is my process path correct?
Thanks, kind regards,
Jay