- #1
gosatomnadzor
- 4
- 0
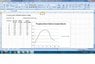
I started with a 100% water sample, and measured the speed of ultrasound using 1MHz transducer, and in 10% increments added propylene glycol to the sample fixture until I reached 100% propylene glycol.
Curiously, the velocity of ultrasound waves when plotted against % propylene glycol resembled a bell curve with the peak velocity at a mixture of 50% propylene glycol / 50% water mixture. See picture attached for graph. Does anyone have an explanation why the velocity increases until 50-50 mixture, then tails off as % propylene glycol increases?
Curiously, the velocity of ultrasound waves when plotted against % propylene glycol resembled a bell curve with the peak velocity at a mixture of 50% propylene glycol / 50% water mixture. See picture attached for graph. Does anyone have an explanation why the velocity increases until 50-50 mixture, then tails off as % propylene glycol increases?