hamurph
- 1
- 0
Good afternoon, all.
Firstly, I apologise if this question is a bit daft, I have just started a nuclear physics course so I am only a baby (I have also been reading ahead so that may be causing most of my problem!)
(I have also been reading ahead so that may be causing most of my problem!)
Looking at the U238 deay chain as an example, I understand that anything with a Protron No. above the line of stability on the Nuclide chart go through Beta+ or electron capture and anything below the line of stability go through Beta- decay.
What is confusing me is how do I predict which ones will go through Alpha decay? Alpha decay occurs from U238 right down to when it stabilises at Pb206!
I have been reading an e-book on atomic and nuclear physics and all it says is "Most Nuclides that will undergo Alpha decay are found in the top Right hand corner of the chart of nuclides" - I was hoping for something like "Where the No. of Neutrons of the isotope exceed the stable isotope's neutrons by X" or something more definite\layman
Thank you anyone who helps me and feel free to laugh and jeer!
Hamurph
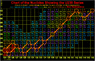
p.s. I am sure you guys are more than familiar, but I have attached a picci of the U238 series because I think it looks nice
Firstly, I apologise if this question is a bit daft, I have just started a nuclear physics course so I am only a baby
Looking at the U238 deay chain as an example, I understand that anything with a Protron No. above the line of stability on the Nuclide chart go through Beta+ or electron capture and anything below the line of stability go through Beta- decay.
What is confusing me is how do I predict which ones will go through Alpha decay? Alpha decay occurs from U238 right down to when it stabilises at Pb206!
I have been reading an e-book on atomic and nuclear physics and all it says is "Most Nuclides that will undergo Alpha decay are found in the top Right hand corner of the chart of nuclides" - I was hoping for something like "Where the No. of Neutrons of the isotope exceed the stable isotope's neutrons by X" or something more definite\layman
Thank you anyone who helps me and feel free to laugh and jeer!
Hamurph
p.s. I am sure you guys are more than familiar, but I have attached a picci of the U238 series because I think it looks nice