Homework Help Overview
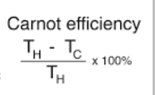
The discussion revolves around the second law of thermodynamics, specifically examining the implications of a hypothetical engine using a reservoir at -5 K. Participants are exploring the concept of efficiency in relation to temperature and questioning the validity of assumptions regarding heat flow between positive and negative temperatures.
Discussion Character
- Conceptual clarification, Assumption checking, Exploratory
Approaches and Questions Raised
- Participants are questioning whether efficiency can be calculated solely based on temperature and discussing the implications of using a negative temperature reservoir. There are inquiries about the definitions of energy terms in the relevant equations and the assumptions about heat flow between reservoirs of differing temperatures.
Discussion Status
Some participants have provided guidance on clarifying the variables in the relevant equations and the need to understand the second law of thermodynamics. There is an ongoing exploration of the implications of negative temperatures and their relationship to positive temperatures, with no explicit consensus reached on the original question.
Contextual Notes
Participants note the requirement to provide their best effort before receiving help, and there is mention of the need for clarity in terminology regarding energy definitions. The discussion also highlights the unconventional nature of thermodynamic temperature and its implications for the problem at hand.
 ##\qquad## !
##\qquad## !