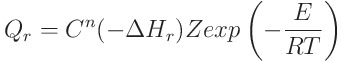Discussion Overview
The discussion revolves around identifying a chemistry equation or formula referenced in the anime Dr. Stone, exploring its applications and interpretations within the context of chemical reactions.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
Main Points Raised
- Some participants suggest that the equation could be a form of the Arrhenius equation, which describes reaction rates.
- Another participant interprets the equation as representing the local rate of heat production per unit volume due to a chemical reaction, relating it to the reaction rate and change in enthalpy, with considerations for exothermic and endothermic reactions.
- One participant points out that an earlier explanation was incorrect, referencing the interpretation provided in a previous post.
Areas of Agreement / Disagreement
Participants express differing interpretations of the equation, with some agreeing on its relation to the Arrhenius equation while others challenge the accuracy of earlier claims. The discussion remains unresolved regarding the correct interpretation.
Contextual Notes
There are indications of missing assumptions and potential dependencies on definitions related to the equation's interpretation. The discussion does not resolve these aspects.