treedwellerjess
- 1
- 0
Net Force in a DNA molecule??
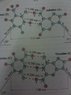
The two strands of the helix-shaped DNA molecule are held together by electrostatic forces as shown in Fig. 16-44. Assume that the net average charge (due to electron sharing) indicated on H and N atoms is 0.2e and on the indicated C and O atoms is 0.4e. Assume also that atoms on each molecule are separated by 1.0 10-10 m
Estimate the net force between each of the following. For each bond (red dots consider only the three atoms in a line (two atoms on one molecule, one atom on the other)).
(a) a thymine and an adenine
(b) a cytosine and a guanine
(c) Estimate the total force for a DNA molecule containing 105 pairs of such molecules.
F=k (q(1)q(2))/d^2 k=8.99E-9
Homework Statement
The two strands of the helix-shaped DNA molecule are held together by electrostatic forces as shown in Fig. 16-44. Assume that the net average charge (due to electron sharing) indicated on H and N atoms is 0.2e and on the indicated C and O atoms is 0.4e. Assume also that atoms on each molecule are separated by 1.0 10-10 m
Estimate the net force between each of the following. For each bond (red dots consider only the three atoms in a line (two atoms on one molecule, one atom on the other)).
(a) a thymine and an adenine
(b) a cytosine and a guanine
(c) Estimate the total force for a DNA molecule containing 105 pairs of such molecules.
Homework Equations
F=k (q(1)q(2))/d^2 k=8.99E-9