Frodo
Gold Member
- 201
- 91
See How we measure background CO2 levels on Mauna Loa for hourly measurements during the day at Mauna Loa which says
Measurement Method
An example of the output from the analyzer for one day is shown in Figure 1 [below]. Two separate intake lines are used for sampling ambient air. The intake lines are from the top of a 38 m tall tower next to the observatory, to avoid any influence on the measurements by human activities at the observatory. Each intake line is measured for five minutes, alternating between line 1 and line 2. Once an hour, the reference gas R0 is measured for five minutes. Once per day, two target tanks are measured for fifteen measurements each (seen in hour 6 and hour 17 in Figure 1). The first few minutes after each gas change are not used to allow time for the previous gas to be completely flushed from the analysis system. The difference of the ambient air measurements from the reference R0 are calculated, and these differences are put into equation 1 to calculate the true ambient mole fraction CO2. By making the measurements relative to the difference from R0, any short term drifts in the analyzer are accounted for.
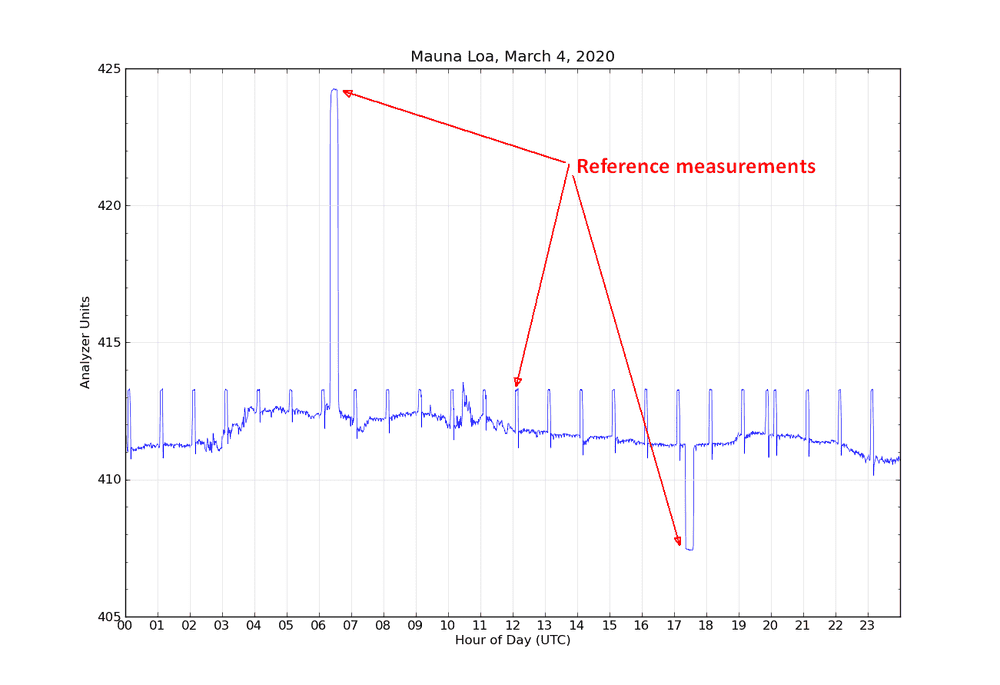
Measurement Method
An example of the output from the analyzer for one day is shown in Figure 1 [below]. Two separate intake lines are used for sampling ambient air. The intake lines are from the top of a 38 m tall tower next to the observatory, to avoid any influence on the measurements by human activities at the observatory. Each intake line is measured for five minutes, alternating between line 1 and line 2. Once an hour, the reference gas R0 is measured for five minutes. Once per day, two target tanks are measured for fifteen measurements each (seen in hour 6 and hour 17 in Figure 1). The first few minutes after each gas change are not used to allow time for the previous gas to be completely flushed from the analysis system. The difference of the ambient air measurements from the reference R0 are calculated, and these differences are put into equation 1 to calculate the true ambient mole fraction CO2. By making the measurements relative to the difference from R0, any short term drifts in the analyzer are accounted for.
