SUMMARY
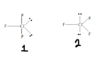
Chlorine trifluoride (ClF3) exhibits a trigonal bipyramidal arrangement and a T-shaped molecular geometry, contrary to the possibility of a trigonal planar shape. According to VSEPR theory, non-bonding electron pairs are sterically more demanding than bonding pairs, necessitating greater spatial separation. In ClF3, the arrangement of non-bonding pairs results in a geometry that minimizes electron pair repulsion, confirming its T-shaped structure rather than a planar configuration.
PREREQUISITES
- Understanding of VSEPR theory
- Knowledge of molecular geometry
- Familiarity with electron pair repulsion concepts
- Basic chemistry terminology related to molecular structures
NEXT STEPS
- Study VSEPR theory in detail
- Research molecular geometry classifications
- Explore the concept of steric hindrance in chemistry
- Learn about electron pair repulsion and its effects on molecular shapes
USEFUL FOR
Chemistry students, educators, and professionals interested in molecular geometry and electron pair interactions will benefit from this discussion.