obiscr
- 3
- 2
- TL;DR
- The problem of nuclei passing through each other.
1. Books and desks have only one atomic core
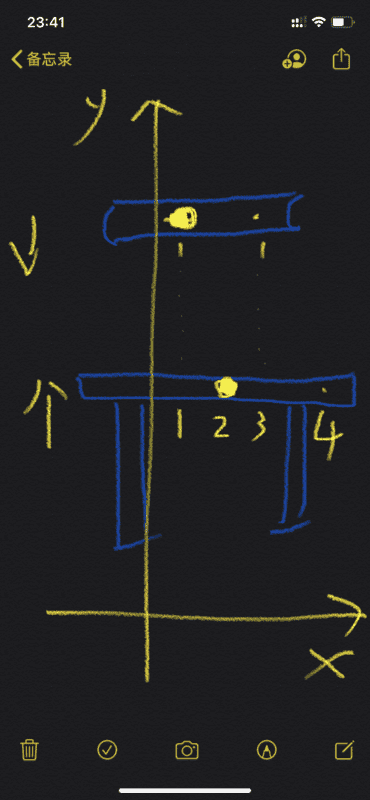
There is a book and desk, suppose the book and desk are composed of atomic atomic core and extranuclear electrons.
Now, the book moves down along the y axis, while the desk stands still or moves up.
Now there is a problem: the position of the x-axis corresponding to the atomic core of the book is 1, and the position of the x-axis corresponding to the outer electron of the book is 3; the position of the x-axis atomic core corresponding to the desk is 2, and the x-axis corresponding to the desk outside the atomic core The position of the electron is 4. In this case, the book and the desk will pass through each other, and the book will not be stably placed on the desk.
The above is when there are few atomic core in books and desks.
2. Books and desks have multiple atomic core
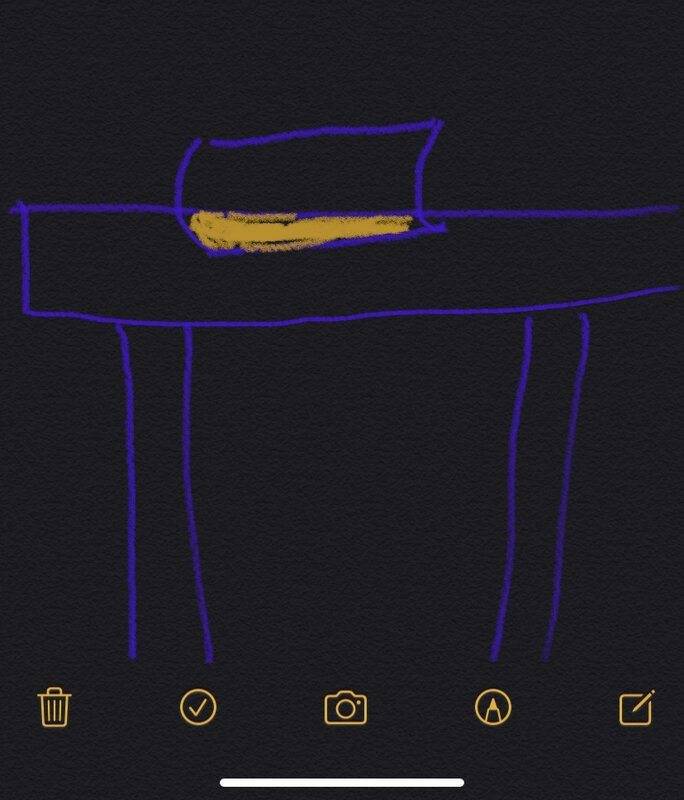
Assuming that there are many atomic core in the book and desk, the possibility of collision between the atomic core in the book and desk is also greater. At this time, this situation will occur: a part of the atomic atomic core collides, and the force generated between the atomic atomic core overcomes the universal gravitation, which makes the book unable to move down along the y axis. The phenomenon is that the book is inlaid on the table. Of course, the depth of the inlays may not be the same, and at the same time they are unlikely to pass through each other.
But what we encounter in the real world is that the book can be stably placed on the table. There will never be a situation where a book is embedded in a table.
I have always been curious about why this is, and I don’t know if anyone can answer this question. Is there a problem with my way of understanding this question?
Thank you!
There is a book and desk, suppose the book and desk are composed of atomic atomic core and extranuclear electrons.
Now, the book moves down along the y axis, while the desk stands still or moves up.
Now there is a problem: the position of the x-axis corresponding to the atomic core of the book is 1, and the position of the x-axis corresponding to the outer electron of the book is 3; the position of the x-axis atomic core corresponding to the desk is 2, and the x-axis corresponding to the desk outside the atomic core The position of the electron is 4. In this case, the book and the desk will pass through each other, and the book will not be stably placed on the desk.
The above is when there are few atomic core in books and desks.
2. Books and desks have multiple atomic core
Assuming that there are many atomic core in the book and desk, the possibility of collision between the atomic core in the book and desk is also greater. At this time, this situation will occur: a part of the atomic atomic core collides, and the force generated between the atomic atomic core overcomes the universal gravitation, which makes the book unable to move down along the y axis. The phenomenon is that the book is inlaid on the table. Of course, the depth of the inlays may not be the same, and at the same time they are unlikely to pass through each other.
But what we encounter in the real world is that the book can be stably placed on the table. There will never be a situation where a book is embedded in a table.
I have always been curious about why this is, and I don’t know if anyone can answer this question. Is there a problem with my way of understanding this question?
Thank you!
Last edited: