- #1
PandaKitten
- 11
- 1
- Homework Statement
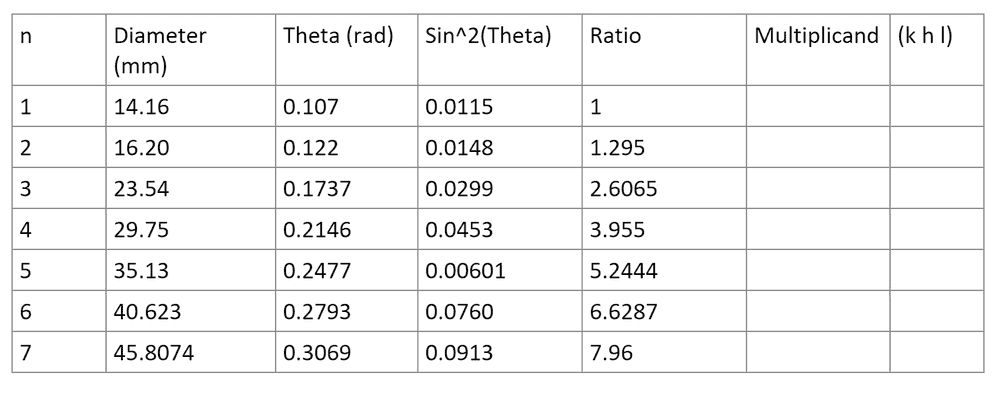
- I need to work out the structure of NaCl using its Debye rings diameter and the equation below by working out its multiplicand and then assigning the appropriate plane structure to it.
- Relevant Equations
- (h^2+k^2+l^2)_min * (sin^2(theta_n))/(sin^2(theta_min)) = integer
Below is the measured values for the Debye rings I obtained. I have to multiply the ratio (which is (sin^2(theta_n))/(sin^2(theta_min))) by a multiplicand until I get an integer. However for the multiplicand and the values I measured I get 1, 3, 13, ??, 4, 8, ??. These should either correspond to a cubic structure (1,2,3,4,5...) a body centric structure (2,4,8,10..) or a face centric structure (3,4,8,11,12,16) but they don't correspond to any of them and also they should be in ascending size.