- #1
hyddro
- 74
- 2
Hello,
I recently did an experiment of nanoparticles (specifically Au Nanoparticles)
We did the Turkievich method and the borohydride method)
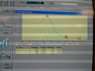
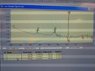
for the turkievich method we got good results and this is indicated by the UV spectrum of the sample..
for borohydride though... we got a funny UV spectrum. I will post both pictures and I need people to help me explain the difference. (since I am not too familiar with UV spectra)
The au colloids from the first method are supposed to be of about 20nm in diameter and the ones from borohydride are supposed to be of about 10nm in diameter.
Also the color of the solution from the second method was gray, which was very confusing.
Finally we tried to aggregate the partticles by using NaCl and ethanol separetly.
for the turkievich colloids, the the solution (whcih was initially dark red) became clear when we used NaCl but didnt change when we added ethanol (we added about 30 drops).
for the borohydride colloids, the solution went from gray to clear when we added nacl and ethanol (Separately) .. i don't know what this means... please any help will be appreciated.
I recently did an experiment of nanoparticles (specifically Au Nanoparticles)
We did the Turkievich method and the borohydride method)
for the turkievich method we got good results and this is indicated by the UV spectrum of the sample..
for borohydride though... we got a funny UV spectrum. I will post both pictures and I need people to help me explain the difference. (since I am not too familiar with UV spectra)
The au colloids from the first method are supposed to be of about 20nm in diameter and the ones from borohydride are supposed to be of about 10nm in diameter.
Also the color of the solution from the second method was gray, which was very confusing.
Finally we tried to aggregate the partticles by using NaCl and ethanol separetly.
for the turkievich colloids, the the solution (whcih was initially dark red) became clear when we used NaCl but didnt change when we added ethanol (we added about 30 drops).
for the borohydride colloids, the solution went from gray to clear when we added nacl and ethanol (Separately) .. i don't know what this means... please any help will be appreciated.