- #1
mzh
- 64
- 0
In the below figure, I'm supposed to express the z-coordinate of the point [itex]P[/itex], [itex]z'[/itex], by the angle [itex]\theta[/itex]
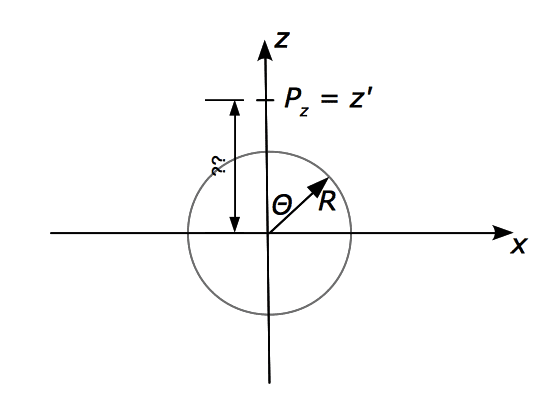
Does this work out as [itex]z' = R\cdot \cos \theta[/itex]? If so, I can't see why...
Please give me a hint on this.
Does this work out as [itex]z' = R\cdot \cos \theta[/itex]? If so, I can't see why...
Please give me a hint on this.