- #1
prehisto
- 115
- 0
Hi,guys.
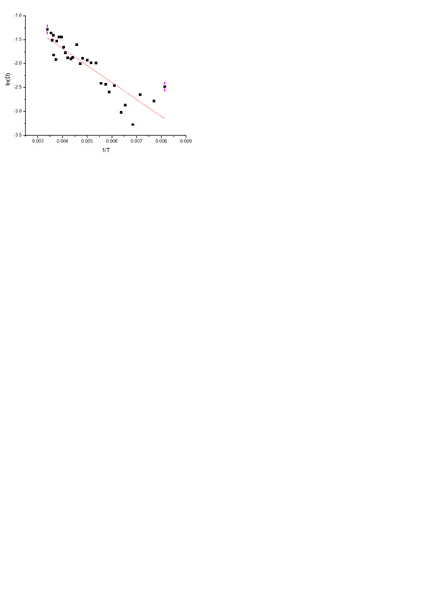
I m trying to determine activation energy of donor levels .
So i have measured absorption at different temperatures of my sample (mono-crystal)
And I have plotted ln(optical density) vs 1/T(K).
And I want to calculate activation energy useing
D=D0exp(-Ea/kT)
In plot above (picture) Eactivation should be 0.043eV
The problem is that I can't get the right answer.
Could someone help me,and derive the mentioned formula above for Ea determination? Maybe I am doing it wrong.
I m trying to determine activation energy of donor levels .
So i have measured absorption at different temperatures of my sample (mono-crystal)
And I have plotted ln(optical density) vs 1/T(K).
And I want to calculate activation energy useing
D=D0exp(-Ea/kT)
In plot above (picture) Eactivation should be 0.043eV
The problem is that I can't get the right answer.
Could someone help me,and derive the mentioned formula above for Ea determination? Maybe I am doing it wrong.