sameeralord
- 659
- 3
Hello everyone,
I'm struggling a little bit to understand the structure of haemoglobin. I have few questions to ask.
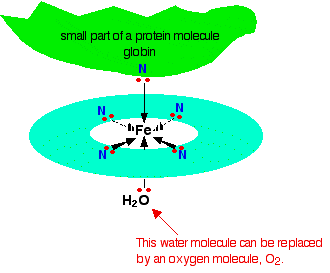
1.Ok so the top bit must be the proximal histidine and in haemoglobin rather than water bottom bit is attached to distal histidine when oxygen is not present right? Now my question is why iron atom able to make 6 bonds. For example if it is oxygen, I know it has 6 electrons in final outershell so it need 2 electrons meaning 2 bonds. (I know energy levels are bit more complicated but this is my level at the moment). Using the same prinicple iron has 26 electrons, that mean it has 16 electrons in its furthest shell so I don't understand where 6 bonds are coming?
2. When the iron atom in heme group is attached to 6 bonds, is it just an iron atom, is it when it has only 4 bonds it becomes ferrous atom (Fe2+). I don't understand where Fe2+ is coming from. This is the picture I got from wikipedia that show heme group? Where is the Fe2+ atom and what is its importance?
[URL]http://upload.wikimedia.org/wikipedia/commons/4/4d/Heme.svg[/URL]
3. Is oxygen attached to the distal end of the histidine as well as the iron atom, or just the iron atom only?
4. What is the difference between tertiary structure and quaternary structure here? Is the 3 dimensional shape of 1 polypeptide chain called tertiary structure and many quaternary structure?
Thank you so much
I'm struggling a little bit to understand the structure of haemoglobin. I have few questions to ask.
1.Ok so the top bit must be the proximal histidine and in haemoglobin rather than water bottom bit is attached to distal histidine when oxygen is not present right? Now my question is why iron atom able to make 6 bonds. For example if it is oxygen, I know it has 6 electrons in final outershell so it need 2 electrons meaning 2 bonds. (I know energy levels are bit more complicated but this is my level at the moment). Using the same prinicple iron has 26 electrons, that mean it has 16 electrons in its furthest shell so I don't understand where 6 bonds are coming?
2. When the iron atom in heme group is attached to 6 bonds, is it just an iron atom, is it when it has only 4 bonds it becomes ferrous atom (Fe2+). I don't understand where Fe2+ is coming from. This is the picture I got from wikipedia that show heme group? Where is the Fe2+ atom and what is its importance?
[URL]http://upload.wikimedia.org/wikipedia/commons/4/4d/Heme.svg[/URL]
3. Is oxygen attached to the distal end of the histidine as well as the iron atom, or just the iron atom only?
4. What is the difference between tertiary structure and quaternary structure here? Is the 3 dimensional shape of 1 polypeptide chain called tertiary structure and many quaternary structure?
Thank you so much
Attachments
Last edited by a moderator:
