FishmanGeertz
- 189
- 0
In chemistry and biochemistry, what do the hexagons with letters in between them mean? Call me silly but for some reason they never taught this to us in school.
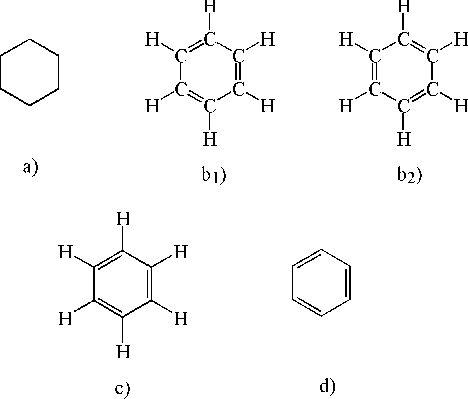
The discussion revolves around the meaning and representation of hexagonal shapes in chemistry, particularly in the context of organic chemistry and molecular structures. Participants explore the significance of these shapes, their notation, and the underlying concepts related to molecular formulas and bonding.
Participants generally agree on the basic definitions and representations of hexagonal shapes in chemistry, but there are differing views on the implications of these representations, such as the role of hydrogen atoms and the uniqueness of skeleton formulas compared to written chemical formulas. The discussion remains unresolved regarding the nuances of these representations.
Some limitations include the assumption that each intersection in the skeleton formula represents a carbon atom, and the potential for confusion regarding the omission of hydrogen in certain representations. The discussion does not resolve the complexities of these notations.
QuarkCharmer said:These shapes are called skeleton formulas. It's a short hand way of representing a molecule. Lines for single bonds, double lines for double bonds etc. It's assumed that each intersection point of the line segments is a molecule, usually carbon, but you can have structures that are not consisting of carbon atoms, these are called heteroatoms and you represent them by noting the symbol for the atom in the chain like this:
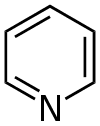
In your posted image, you would assume (since there is no notation) that each point is a carbon atom. Interestingly enough, the only way they can maintain that hex form is by having alternating double bonds, hence the alternating double lines in your image. Hope that helps. I am sure you can find more info now that you know what they are called.