You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Acid: Definition and 698 Discussions
An acid is a molecule or ion capable of either donating a proton (i.e., hydrogen ion, H+), known as a Brønsted–Lowry acid, or, capable of forming a covalent bond with an electron pair, known as a Lewis acid.The first category of acids are the proton donors, or Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Arrhenius acids. Brønsted and Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+.
Aqueous Arrhenius acids have characteristic properties which provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and react with bases and certain metals (like calcium) to form salts. The word acid is derived from the Latin acidus/acēre, meaning 'sour'. An aqueous solution of an acid has a pH less than 7 and is colloquially also referred to as "acid" (as in "dissolved in acid"), while the strict definition refers only to the solute. A lower pH means a higher acidity, and thus a higher concentration of positive hydrogen ions in the solution. Chemicals or substances having the property of an acid are said to be acidic.
Common aqueous acids include hydrochloric acid (a solution of hydrogen chloride which is found in gastric acid in the stomach and activates digestive enzymes), acetic acid (vinegar is a dilute aqueous solution of this liquid), sulfuric acid (used in car batteries), and citric acid (found in citrus fruits). As these examples show, acids (in the colloquial sense) can be solutions or pure substances, and can be derived from acids (in the strict sense) that are solids, liquids, or gases. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.
The second category of acids are Lewis acids, which form a covalent bond with an electron pair. An example is boron trifluoride (BF3), whose boron atom has a vacant orbital which can form a covalent bond by sharing a lone pair of electrons on an atom in a base, for example the nitrogen atom in ammonia (NH3). Lewis considered this as a generalization of the Brønsted definition, so that an acid is a chemical species that accepts electron pairs either directly or by releasing protons (H+) into the solution, which then accept electron pairs. However, hydrogen chloride, acetic acid, and most other Brønsted–Lowry acids cannot form a covalent bond with an electron pair and are therefore not Lewis acids. Conversely, many Lewis acids are not Arrhenius or Brønsted–Lowry acids. In modern terminology, an acid is implicitly a Brønsted acid and not a Lewis acid, since chemists almost always refer to a Lewis acid explicitly as a Lewis acid.
View More On Wikipedia.org
Aqueous Arrhenius acids have characteristic properties which provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and react with bases and certain metals (like calcium) to form salts. The word acid is derived from the Latin acidus/acēre, meaning 'sour'. An aqueous solution of an acid has a pH less than 7 and is colloquially also referred to as "acid" (as in "dissolved in acid"), while the strict definition refers only to the solute. A lower pH means a higher acidity, and thus a higher concentration of positive hydrogen ions in the solution. Chemicals or substances having the property of an acid are said to be acidic.
Common aqueous acids include hydrochloric acid (a solution of hydrogen chloride which is found in gastric acid in the stomach and activates digestive enzymes), acetic acid (vinegar is a dilute aqueous solution of this liquid), sulfuric acid (used in car batteries), and citric acid (found in citrus fruits). As these examples show, acids (in the colloquial sense) can be solutions or pure substances, and can be derived from acids (in the strict sense) that are solids, liquids, or gases. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.
The second category of acids are Lewis acids, which form a covalent bond with an electron pair. An example is boron trifluoride (BF3), whose boron atom has a vacant orbital which can form a covalent bond by sharing a lone pair of electrons on an atom in a base, for example the nitrogen atom in ammonia (NH3). Lewis considered this as a generalization of the Brønsted definition, so that an acid is a chemical species that accepts electron pairs either directly or by releasing protons (H+) into the solution, which then accept electron pairs. However, hydrogen chloride, acetic acid, and most other Brønsted–Lowry acids cannot form a covalent bond with an electron pair and are therefore not Lewis acids. Conversely, many Lewis acids are not Arrhenius or Brønsted–Lowry acids. In modern terminology, an acid is implicitly a Brønsted acid and not a Lewis acid, since chemists almost always refer to a Lewis acid explicitly as a Lewis acid.
View More On Wikipedia.org
-
J
Biochemistry: pH of L-glutamic acid
Homework Statement Calculate the pH of a solution of L-glutamic acid in which the ionic species with a net charge of -1 is 5 times greater than that of the isoelectric structure. (Refer to pKa Table at end of problem set). Homework Equations pKa for glutamic acid: -COOH = 2.19...- jake222
- Thread
-
- Tags
- Acid Biochemistry Ph
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
R
How much base to neutralize acid?
I have a question in my environmental engineering class that goes like this: if 100mg H2SO4 is added to 1 Liter of water, how many milligrams of NaOH (strong base) must be added to neutralize the acid? the answer is 81.6mg, but how do i get to the answer? i tried writing a reaction of...- ride5150
- Thread
-
- Tags
- Acid Base Neutralize
- Replies: 1
- Forum: Chemistry
-

What is the impact of long-term storage on sealed lead acid batteries?
I am offered Sealed Lead acid batteries at heavy discount (half the price) at local Sale. But they were manufactured some 5 years back. How much damage does long storage do to them? They have never been touched. Just bought 5 years back, and stored.- I_am_learning
- Thread
- Replies: 4
- Forum: Electrical Engineering
-
H
Are all acid have covalent structure in solid state?
Are all acid have covalent structure in solid state? -
T
So how do you know when a chemical is an acid or not? I know that an
So how do you know when a chemical is an acid or not? I know that an acid is a compound that, when dissolved, yields H atoms, but does that mean than any compound with H in it is (or has the potential to be) an acid? For example: HNO2, I put down that it was Hydrogen Nitrite, but the...- Titandwedebil
- Thread
- Replies: 4
- Forum: Chemistry
-
P
Is SiO3 soluble in Hydrofluoric acid?
Is SiO3 soluble in Hydrofluoric acid? Is SiO3 soluble in Hydrofluoric acid? Why am I asking this: I have some crystals of Be3Al2(SiO3)6 - Beryl and there are structural channels which I want to enlarge...! (I know it sounds impossible) Here is how the crystal's structure looks like...- Panthera Leo
- Thread
-
- Tags
- Acid
- Replies: 5
- Forum: Chemistry
-
S
Why is sulphuric acid added to copper sulfate solution for electroplating?
Electroplated a 5 micron layer of copper a few days ago. Was wondering why there's sulphuric acid in the solution.- sgsawant
- Thread
-
- Tags
- Acid Copper Electroplating
- Replies: 1
- Forum: Chemistry
-
A
Citric acid buffer solution for prac
Hi Guys, Citric acid has three different values for pKa because it is a polyprotic acid. I am aiming to make a buffer solution with sodium citrate but I am not sure which pKa value to use in the Henderson-Hasselbalch equation. Does anyone have any ideas? -
N
Why are acidic amino acid side chains acidic?
I have been trying to work this out over 2 days now and I just don't understand! The side chains of Aspartic acid and Glutamic acid both have the reactive group COO-. I don't understand how the COO- is an acidic group! To me, I can see how it could be a basic group - the H+ binds to the COO-...- nucleargirl
- Thread
-
- Tags
- Acid
- Replies: 4
- Forum: Chemistry
-
B
Determine volume of HCl added to propionic acid to get pH=1
Homework Statement Given 250ml of 0.1M HC3H5O2 (Ka=1.35E-5) What volume of a)1.0M HCl is needed to lower the pH to 1.00. b) 1.00M NaC3H5O2 raise pH to 4.00 Homework Equations pH=pKa-log (HA/A) ICE chart The Attempt at a Solution I used the ice chart for the dissociation of...- BThomas1219
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
A
Change in ascorbic acid over time
Why does ascorbic acid change in concentration over time?- Amili
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
Charging a lead acid battery
I want to charge a led-acid battery it needs 12V DC 1.i have transformer to convert 220V AC to 12V AC after that can i give 12V AC to bridge rectifier and from bridge rectifier to battery terminals directly or should i use a filter to remove the fluctuations in DC ? 2. what should be the... -
N
Stearic acid / magnesium stearate in vitamins? Is it safe to consume?
Does stearic acid do bad things for the human body? Apparently stearic acid is in most vitamins if you take vitamins, it says it in the ingredient list, most people until now it seems did not even know that it is bad for you, but no other article anywhere else on the web says this except for one... -
N
Does Salicylic Acid Work by Hydrolyzing to its Active Form?
Hi guys, I just have a quick query about the mechanism of action of salicylic acid. I have tried in vain to find a solution elsewhere, but have not had any luck. How exactly does salicyclic acid work. As far as I can tell, it doesn't have an acetyl group, which is how aspirin mediates its...- nymbler_064
- Thread
-
- Tags
- Acid
- Replies: 2
- Forum: Chemistry
-
D
Thermal Properties of Sulphuric Acid Anodic Coatings
Hi, Can anyone tell me at what temperature a 25micron sulphuric acid anodic coating will start to breakdown. I have noticed that after exposure to 135°C the electrical properties of the coating have dropped drastically. Regards Dan- dantyco
- Thread
-
- Tags
- Acid Properties Thermal
- Replies: 1
- Forum: Materials and Chemical Engineering
-
N
Function of Acetic Acid in Aspirin
I was just wondering if anyone would be able to tell me why acetic acid is reacted with salicylic acid to form aspirin. I am aware that the latter is toxic and that the addition of acetic acid lessens its effects, but what specific properties does acetic acid have that enable it to do so. I have...- nymbler_064
- Thread
- Replies: 9
- Forum: Chemistry
-
Lifetime of rechargeable battery (sealed lead acid)
I have a cordless electric lawnmower that runs on sealed lead acid batteries. It is on it's second battery, which has noticeable degraded performance and will need replacing soon. My question is why the original battery, which was rated at 17 Amp-hours, lasted twice as long as this second...- Redbelly98
- Thread
- Replies: 4
- Forum: Chemistry
-
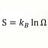
Maximum concentration, molarity, of Carbonic Acid ?
What is the maximum concentration, Moles/liter, possible for one liter of H2CO3 at STP. And how is that calculated ? -
P
Dissolving an Unknown Acid Salt - Weak Acids?
i'm trying to dissolve an unknown acid salt and it is not soluble in water at stp. it does dissolve, however, when it undergoes heat and some stirring. is this property representative of most weak acids, that they are not easily miscible in water? -
E
Why does a lead acid battery spark when connecting to opposite terminals
Sorry about the intrusion as I really don't belong here as I have absolutely no background in physics but I have a couple of questions regarding lead acid batteries that I have been unable to find answers to on the internet. First question - why does a battery spark/arc when the negative is...- Edible Planet
- Thread
- Replies: 4
- Forum: Other Physics Topics
-
E
Fatty Acid chain length and its effect on binding to serum albumin
Homework Statement The background: We performed an experiment testing the fatty acid binding difference of caproic (hexanoic) acid and lauric (dodecanoic) acid to bovine serum albumin. The experiment was performed using a fluorescent marking called ANS(which fluoresces when bound to bovine...- Eshi
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
G
What Volume of Ethanoic Acid is Needed to Neutralize 25ml of 0.1 M NaOH?
Hi, I came across a problem, which I might have solved a few years ago when I had studied Chemistry, but now I am totally stumped. Would appreciate some quick solutions. I have 25 ml of 0.1 M NaOH, and a volume of 0.1 M of Ethanoic Acid. What volume of ethanoic acid would neutralize the 25... -
S
Weak Acid - Strong Base titration. Too little information?
Homework Statement In order to achieve a high result in this chemistry assignment, I need to have demonstrated a knowledge of challenging content not discussed in class. Every other part of this assignment I've covered (Intro, method, discussion etc), I am just stuck with the calculations...- stickynote
- Thread
-
- Tags
- Acid Base Information Titration Weak
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
Q
Can Temperature Affect the Dissociation of Strong Acids?
Hi Everyone, I was just wondering if you know of any factors that would affect the dissociation of a strong acid. I know that weak acids are affected by factors such as the common ion effect and temperature, but would this apply to strong acids as well? I only ask because we added...- qwerty1232
- Thread
-
- Tags
- Acid Dissociation
- Replies: 16
- Forum: Chemistry
-
R
Titration of unknown acid in lab, how to determine molar mass?
Hi, Homework Statement It is in my chemistry lab course. I had 0.1g of an unknown solid that I diluted in 100ml of distilled water. Then, I took 25ml and titrated it with NaOH (Concentration = 0.0189M). To reach equivalent point (the point where concentration of NaOH is equal to the...- redioactif
- Thread
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
O
Finding Ka of Monoprotic Acid using information from titration
1. This problem has been slowly eating me for the past 2 hours. I've done everything I can but I can't seem to answer it. The pH at the equivalence point in the titration of 100 mL of a 0.1M monoprotic acid solution with a 0.1M strong base solution is 8.12 at 25 degrees C. What is the Ka of the...- Orims
- Thread
-
- Tags
- Acid Information Titration
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
D
From hydrochloric acid to hydrogen chloride
It is easy to produce hydochloric acid from hydrogen chloride by simply dissolving it into water or even with room humidity, it is sufficiently to form dense white fumes of hydrochloric acid. But is it possible to produce or extract hydrogen chloride from hydrochloric acid? (At best, a cheap... -
S
What vessels to use with hydrochloric acid, sodium hydroxide, and sulfuric acid?
Hello. I am in need of some assistance in regards to what types of materials I should (and should not) use to contain the various corrosive substances listed in the title. As for the sodium hydroxide, it will be dissolved in water, almost to the point of saturation. I have never dealt with...- SMD1990
- Thread
-
- Tags
- Acid Sodium Sodium hydroxide
- Replies: 1
- Forum: Chemistry
-
S
Strontium nitrate and sulfuric acid equation
Find the molecular, total ionic, and net ionic equation for the aqueous reaction between strontium nitrate and sulfuric acid. I did like this, please check this out... Sr(NO3)2 + H2SO4 -> 2HNO3 + SrSO4 is molecular equation. Sr+2(aq) + 2NO3-1(aq) + H+1(aq) + HSO4-1(aq) -> 2H+1(aq) +...- sunbuster
- Thread
-
- Tags
- Acid
- Replies: 7
- Forum: Biology and Chemistry Homework Help
-
T
Chemistry Molecular weight of unknown acid through titration
Homework Statement The molecular weight of a monoprotic acid HX was to be determined. A sample of 15.126 grams of HX was dissolved in distilled water and the volume were brought to exactly 250mL in a volumetric flask. Several 50mL portions of this solution were titrated against NaOH solution...- The_Journey
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-

Understanding Acid Solutions: How to Reach 10^-14
hello everyone, i read it somewhere:-- In such a solution at 25C, the product of concentration of H+ ions with the concentration of OH- ions must be 10^-14. In an acid solution, by forcing a high concentration of H+ ions, you necessarily suppress the concentration of OH- ions. what is...- PainterGuy
- Thread
-
- Tags
- Acid
- Replies: 1
- Forum: Chemistry
-
D
Determining volume of an acid needed to react with a mass
1. Determine the volume of 1.50 mol dm–3 of hydrochloric acid that would react with exactly 1.25 g of calcium carbonate. 2. 2HCl(aq) + CaCO3(s) --> CaCl2(aq) + CO2(g) + H2O(1) 3. I tried to do this: (1.25 g CaCO3) / (100 g CaCO3) = 0.0125 moles of CaCO3 2 moles of HCl are needed...- Deluxe489
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
A
Dissociation constant of weak acid
I'm preparing for a chemistry lab relating to "Determination of the dissociation constant of a eak acid". In part of the lab, I will b doing the following for measuring "Ka" for ethanoic acid using the indicator method: 1- Mix ethanoic acid (0.02M, 5cm3) and sodium ethanoate (0.02M, 5cm3)...- Abder-Rahman
- Thread
-
- Tags
- Acid Constant Dissociation Weak
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
E
Can 18M Hydrochloric Acid Cause Permanent Skin Damage?
Just a question that is been nagging me for a long time... if a person were to put 18 mol/L HCL(aq) on your skin what kind of damage would there be? weither it be short term effects, long term effects, or just general damage? Replys are welcome :P -
C
Calculating the pH of 350L of Sulfuric Acid (H2SO4)
Homework Statement Sulfuric acid (H2SO4) were collected from the waste acid container and the volume is 350 l. Homework Equations a) Calculate the acid pH of the solution when the hydrogen ion concentration is 2.0 ∙ 10-4 mol / l. The Attempt at a Solution i think it's simple no ...- chawki
- Thread
- Replies: 40
- Forum: Biology and Chemistry Homework Help
-
T
Molarity of Copper Ion solution produced from Copper, Nitric Acid, and Water
Homework Statement (c) A 0.1036 g sample of copper metal is dissolved in 48 mL of concentrated HNO3 to form Cu2+ ions and then water is added to make a total volume of 208.1 mL. (Calculate the molarity of Cu2+.) Homework Equations Molarity = Moles/Liter The Attempt at a Solution...- torquemada
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
C
Zinc Reacting with Hydrochloric Acid: STP Conditions
Homework Statement Base metal zinc (Zn) reacts with concentrated hydrochloric acid (HCl) forming gaseous hydrogen and zinc chloride. STP conditions: pressure p = 101,3 kPa , temperature t = 0C. The equation of state for ideal gas: pV = nRT R = 8,314 J /(mol K) Homework Equations Write...- chawki
- Thread
-
- Tags
- Acid Conditions Zinc
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
R
Determine the molarity of NaOH used to titrate acetic acid.
Homework Statement After titrating 25mL 0.1M acetic acid with NaOH, determine the molarity of the NaOH. Equivalence point: 23.99mL NaOH added, pH = 8.37 Initial point: 0mL of NaOH added, pH = 3.07. Homework Equations ka = kw/kb kb = [CH3COOH][OH]/[CH3COO] The Attempt at...- rss14
- Thread
- Replies: 6
- Forum: Biology and Chemistry Homework Help
-
L
Acid Stripping - with Ultrasonic Assist
Hello PF, I am trying to chemically strip a metal oxide film from fused silica. The film was deposited at a fairly high temperature, which makes it more difficult to strip than at lower temperatures. During deposition, the film makes its way into the pores of the substrate and slow cooling...- lewdtenant
- Thread
-
- Tags
- Acid Ultrasonic
- Replies: 1
- Forum: Chemistry
-
A
Chemistry, acid base titration lab
Homework Statement Assuming stomach acid is 0.1 M HCl(aq), what volume of stomach acid can be neutralized by one antacid tablet? We have the titration curve we got with .5M HCl titrating a tablet dissolved in 100ml distilled water Homework Equations ? The Attempt at a Solution ? -
S
What does one carbon metabolism of folic acid mean?
What does that mean. Transfer of 1-carbon unit. What is a 1-carbon unit, are they referring to carbon number 1(according to nomenclature) of the molecule being transferred or a single carbon atom from the molecule is transferred? Can anyone explain. Thanks :smile:- sameeralord
- Thread
-
- Tags
- Acid Carbon Mean Metabolism
- Replies: 1
- Forum: Chemistry
-
N
How to find the ph in a strong base weak acid titration?
Homework Statement If .0200L of .100M nitrous acid is titrated using .200M NaOH solution, what will the pH be at equivalence? Homework Equations The Attempt at a Solution I figured out that the volume of NaOH at equivalence is .01L, however I do not know what to do after that... -
S
Calculating the Neutralization of Acid in Grapefruit Juice
Homework Statement Grapefruit juice contains approximately 0.75g/oz of citric acid (H3C6H5O7(aq)), a triprotic acid. If one tablet of Tums contains 500mg of CaCO3, would one tablet be able to neutralize the acid in one glass of grapefruit juice (8.0oz)? Show your calculations. Therefore I...- sunfire249
- Thread
-
- Tags
- Acid
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
S
Why Can Dimethyl Ether Extract Benzoic Acid But Dioxane Cannot?
Homework Statement Why is it possible to use dimethyl ether as a solvent for extraction of benzoic acid and not dioxane? Homework Equations The Attempt at a Solution I'm thinking that it's because dioxane has more of a hydrophobic character since it has many carbons, but then...- schoolboy10
- Thread
-
- Tags
- Acid Extraction
- Replies: 11
- Forum: Biology and Chemistry Homework Help
-
E
Solubility of acetylsalicyclic acid in ethyl acetate
Hi I am looking for the solubility of acetyl salicyclic acid in ethyl acetate vs. temperature. So far I have only been able to find its solubility at 25 degrees C (0.453 M). However, I would like to know its solubility at higher temperatures (77 degrees C, to be precise). If anyone knows...- espen180
- Thread
-
- Tags
- Acid Solubility
- Replies: 1
- Forum: Chemistry
-
L
How does formic acid (under basic conditions) convert to CO2?
What is the mechanism that results in formic acid quickly converting to CO2. I know this happens under basic conditions so I'm assuming the first step is forming the formate ion. -
J
Lactic acid vs lactate in sweat
I want to measure the pH of sweat in order to estimate the anaerobic threshold by detection of lactic acid. Many references point out that it's lactate, not lactic acid, that is found in sweat. However, according to this article...- juanfhj
- Thread
-
- Tags
- Acid
- Replies: 4
- Forum: Biology and Medical
-
O
Compare pH of HNO3 & HBr: Which is Stronger Acid?
which acid is more strong HNO3 or HBr? i get the the pH for HNO3 is 5 and for HBr is 2 is it Possible if HNO3 is more strong acid so it must have the low pH than HBr yes? thanks- omni
- Thread
- Replies: 10
- Forum: Biology and Chemistry Homework Help
-
S
What is the best problem to solve for an IB Design Lab on acids and bases?
I need some help with developing an idea for my design lab. it is a lab where we must come up iwth a problem to solve on our own. This time we are to discover something new about acids and bases. What would be a good problem? thank you! -
O
Which substances in this equation are acid and base?
i need to mark all the Base and Acid in the Equation (in the picture) and tell why i think i choose Them to be acid and base i mark the Acid in red line and the Base in blue line, tell me if i Right. my Explanation is HNO is Acid and it give proton While CH3NH2 is base and it gain in the...- omni
- Thread
- Replies: 8
- Forum: Biology and Chemistry Homework Help