You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Process: Definition and 1000 Discussions
A process is a series or set of activities that interact to produce a result; it may occur once-only or be recurrent or periodic.
Things called a process include:
View More On Wikipedia.org
Things called a process include:
View More On Wikipedia.org
-
D
What is the Induction Process for Finding det(alpha*A)?
Old topic: https://www.physicsforums.com/showthread.php?t=194725 I have a question that's the same as the one in the old topic/thread I linked to above. At the last post, I am trying to figure out how to carry out the induction process. \sum_{j=1}^n (-1)^{i+j} a_{ij} det(\alpha A_{ij})...- DMOC
- Thread
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-
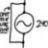
Solving BJT Circuits: A Step-by-Step Guide
Homework Statement I am just curious whether or not there is a step-by-step algorithm that will always produce the desired results when solving BJT circuits? We are learning them in my electronics course at the moment, and it seems as though the approach to the problems is never...- jegues
- Thread
-
- Tags
- Process
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
N
Spatial statistics - Point process on a network of one-dimensional lines
Hi everyone, My data consists of road accidents and spatial covariates. Originally i wanted to apply an inhomogenous Poisson process with covariates. Then I realized that the accidents cannot occur everywhere in space, but only on roads. I found an example, that is quite similar to mine. it...- Nemorad
- Thread
-
- Tags
- Lines Network Point Process Statistics
- Replies: 7
- Forum: Set Theory, Logic, Probability, Statistics
-
B
Isothermal, adiabatic, isovolumetric process
Homework Statement Air with the mass of 10kg, with pressure 15 bar and temperature 50 C comes to a isobaric expansion to 3 times the original volume, then the air cools to the pressure of 6 bar(isovolumetricly). After that it comes to adiabatic expansion to original temperature (50 C i guess)...- Bassalisk
- Thread
-
- Tags
- Adiabatic Isothermal Process
- Replies: 6
- Forum: Advanced Physics Homework Help
-
S
Definition of a reversible process
I have seen a reversible process defined as one in which the system and surroundings are restored to their initial state without change elsewhere. As far as I am aware, the system and the surroundings completely occupy the universe. So, I am failing to understand what elsewhere means in this...- spaghetti3451
- Thread
- Replies: 9
- Forum: Classical Physics
-
B
Ideal gas undergoes cycle process
Hi, An amount of ideal gas undergoes the folowing cycle process in the following graph: This process can be presented by the following PV-graph : The answer has to be the upper graph but why can't it be the lower graph ? Thank you! -
E
Solving Isothermal Process: Heat Supplied at Constant Pressure & Volume
Homework Statement I have a diagram of a process of a monoatomic gas. and the problem is that I know that the total amount of work = total amount of heat, so there is no internal energy change. why? because my process started at the same temp that it finished. how ever... it is asking me...- eliassiguenza
- Thread
-
- Tags
- Isothermal Process
- Replies: 1
- Forum: Introductory Physics Homework Help
-
I
Medical Kidney's process of reabsorpting the water
how could the process of the kidney's process of reabsorpting the water be simulated through a man made experiment?- isyang94
- Thread
- Replies: 1
- Forum: Biology and Medical
-
A
Work done for an adiabatic process
Hi, In deriving the equation for adiabats : V^{\gamma}P = C. It is assumed that the work done during the process is W = -P \Delta V. But calculating the work done from V^{\gamma}P = C we obtain: W = \frac{C}{1-\gamma}\left[ V^{1-\gamma}_{f} - V^{1-\gamma}_{i} \right] . Also, i believe to... -
X
Why is the change in enthelpy 0 during an isothermal process?
I am reviewing material for my thermodynamics class and one of the practice exams says that the change in enthalpy during an isothermal process is equal to zero. This doesn't make any sense to be considering.. \Delta H = \Delta U + PV and since this is an isothermal process.. \Delta U =...- Xyius
- Thread
-
- Tags
- Change Isothermal Process
- Replies: 12
- Forum: Thermodynamics
-
T
Difference between Renewal Process and Poisson Processes
Hey All, Can someone please explain to me the difference between a Poisson Process and a Renewal Process ? is it just that the Holding times for Poisson processes are exponential and Holding times for Renewal Processes are any kind of probability distribution (as the wiki page seems to imply)...- thrillhouse86
- Thread
-
- Tags
- Difference Poisson Process
- Replies: 1
- Forum: Set Theory, Logic, Probability, Statistics
-

Looking for the name of a process.
Suppose we have two Gaussian distributions with means A and B. These systems have probability P at time T to be located at X. These are the probability distributions of a simple one-dimensional Wiener process. M=mean P(M,T)=1/sqrt(2*pi*T) * exp(-(X-M)^2/(2*T)) So: P(A...- Rlam90
- Thread
-
- Tags
- Process
- Replies: 1
- Forum: Quantum Physics
-
R
Interviewing process for graduate physics programs
I was recently invited to be flown to one of the graduate physics programs that I applied to, to be interviewed for a fellowship award (acceptance pending and likely depending on this). I'm fairly excited about this, although I'd like to know what my chances are of being accepted into the...- ranfea
- Thread
- Replies: 1
- Forum: STEM Academic Advising
-
W
Reactor volume in anaerobic biodegradation process
hello, today I faced a problem with explaining why in anaerobic process the volume of UASB reactor is smaller than volume in CSTR with the same amount of final product - biogas Answer should contain characteristics like: difference in kinetics, concentration, rxn yield. Can anybody explain...- War-Saw
- Thread
- Replies: 1
- Forum: Materials and Chemical Engineering
-
D
Supercooled water freezes. Is this a spontaneous process?
Supercooled water freezes. Is this a spontaneous process?? Homework Statement "one mole of supercooled water at -10degC freezes reversibly to ice at -10degC at constant P of 1500atm. The system is at chemical equilibrium. Assume that ΔH_fusion is independent of temperature. Also assume that...- Dars
- Thread
-
- Tags
- Process Spontaneous Water
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
S
Making New Paper Process and Making Recycled Paper Process
What is the main different within them process ? Any good web or source can gain more knowledge about them?- soonsoon88
- Thread
- Replies: 8
- Forum: Other Physics Topics
-
A
Where Can I Find Free Process and Reaction Simulation Software?
Hi, Do anyone know where to get free process simulation software, please, if you know let me know, thanks. -
F
Electrostatic energy in annihilation process
When an electron and a positron annihilate, they typically produce two gamma rays, each of energy mc^2 plus whatever kinetic energy available before annihilation. I was recently told that it is an experimental fact that the electrostatic energy between the electron and the positron does NOT...- FredMadison
- Thread
- Replies: 7
- Forum: Quantum Physics
-
V
How Does Constant Pressure Affect Heat Transfer in Oxygen?
Homework Statement Suppose 15.2 g of oxygen (O2) is heated at constant atmospheric pressure from 13.3°C to 148°C. (a) How many moles of oxygen are present? (Take the molar mass of oxygen to be 32.0 g/mol) (b) How much energy is transferred to the oxygen as heat? (The molecules rotate but do...- VitaX
- Thread
- Replies: 6
- Forum: Introductory Physics Homework Help
-
F
Entropy change of an irreversible process
i can't seem to get something in classical thermodynamics entropy is a state function of a system,that means it only depends on the state of a system not time or path. if i have an irreversible process between state 1 and state 2, and back to state 1, and i want to know the entropy change of...- flasherffff
- Thread
-
- Tags
- Change Entropy Irreversible Process
- Replies: 2
- Forum: Thermodynamics
-
0
Relative Motion - What is your thought process?
This is not a homework question. I know the answer to this question I'm just not sure how, from reading the question, I would have known what the question is actually asking. If someone could explain what their thought process is after reading the question, and how it is that you know what the...- 03125
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
M
Thermodynamics problem- isobaric process
Homework Statement One mole of an ideal monatomic gas is taken through the cycle ABCA shown schematically in the diagram. State A has volume 0.0208 m3 and pressure 1.22 × 10 5 Pa, and state C has volume 0.0524 m3 Process CA lies along the 305 ±1 K isotherm. The molar heat capacities for...- mrdith007
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
C
Thermodynamics - Adiabatic Process
Homework Statement A cylinder, A, containing a monatomic ideal gas, is fitted with a frictionless leakfree piston. The axis of the cylinder is oriented vertically, as in the figure below, so that the weight of the piston maintains the gas at a constant pressure P0. The cylinder is linked...- catdog90210
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
Z
Irreversible adiabatic process
What is the concept behind 'irreversible adiabatic process' ? Why is the expression for work done in this case different from that when it is reversible? -
C
Stochastic Process - Creating a Probability Transition Matrix
Homework Statement The total population size is N = 5, of which some are diseased and the rest are healthy. During any single period of time, two people are selected at random from the population and assumed to interact. The selection is such that an encounter between any pair of individuals...- cse63146
- Thread
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-
D
Medical Opponent process theory: why do I see green after looking at white?
This morning, I was lying on my bed, looking at the bright morning light coming in through the window. The blinds (which are white) were at least half shut, so much of the light was filtered through them. I then stared up at the ceiling, and saw a nebulous patch of green. I shut my eyes, and...- DeuteriumDude
- Thread
- Replies: 4
- Forum: Biology and Medical
-
R
Finding the relesed energy in decay process
Hi, Radioactive materials will decay to datughter products. in the mean process they emit alpha or beta particles with some energy and then decay to new products. In my problem i am just observing U(238) decay process. I need to find the energy values for each decay and also i need...- rama1001
- Thread
- Replies: 2
- Forum: High Energy, Nuclear, Particle Physics
-
K
Is There Any Reversible Quasi-Static Process?
Just out of curiosity, is there any quasi-static process that is completely reversible? I think the answer is an obvious no because any quasi-static process experiences a net energy loss but I'd like to know if there are any, possibly something abstract rather then mechanical.- Kevin_Axion
- Thread
-
- Tags
- Process Quasi-static
- Replies: 2
- Forum: Classical Physics
-
J
The process involved in reflection?
when we have light reflecting off a material, what's going on there? is it something analogous to a gas particle rebounding off a container wall due to Coulomb repulsion? If so, what is it that exerts a force to reverse the photon's momentum perpendicular to the surface it's reflecting off...- jeebs
- Thread
-
- Tags
- Process Reflection
- Replies: 10
- Forum: Quantum Physics
-
V
Adiabatic Process and the speed of sound
Hi all, Sorry if this post is a bit wordy but I've been going round in circles and I thought I'd see if anyone on here can help, it's also my first post so be nice... I've been trying to understand how Newton miscalculated the speed of sound. I know that he thought that the propagation of...- veryownjustin
- Thread
- Replies: 4
- Forum: Mechanics
-
R
How to calculate the heat added during an isothermal process
Homework Statement The titleHomework Equations ?The Attempt at a Solution The only ways I know of to calculate heat are dQ = Cp.dT and dQ = Cv.dT for isochoric and isobaric process how would you go about doing it for an isothermal process Also if a system isn't a cycle but it ends up in the...- renlok
- Thread
-
- Tags
- Heat Isothermal Process
- Replies: 1
- Forum: Advanced Physics Homework Help
-
S
Thermodynamics 1st Law: reversible process for batteries?
How could you ensure that an electric battery produced an electric current reversibly? How could you achieve maximum work from an electric battery? I really have no idea how to solve this. Thanks for your help! (Also sorry I first posted this in advanced physics...i don't know how to...- sparkle123
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
J
Feynman rules and decay process
Hi I need help to understand Feynman rules for decay for exams. In past paper there is the following question as whether the following are allowed. cc decays to tau++tau-. From What I can see this is possible via the strong force is this correct? The next is cc decays to cu and cu. From...- jc09
- Thread
- Replies: 3
- Forum: High Energy, Nuclear, Particle Physics
-
Y
Rules for gaussian random variables - ornstein uhlenbeck process
Homework Statement The Langevin equation for the Ornstein-Uhlenbeck process is \dot{x} = -\kappa x(t) + \eta (t) where the noise \eta has azero mean and variance <\eta (t)\eta (t')> = 2D(t-t')\delta with D \equiv kT/M\gamma. Assume the process was started at t0 = - \infty. Using...- yaboidjaf
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
U
Adiabatic process thermodynamics help
Homework Statement An ideal diatomic gas at 75 K is adiabatically compressed to 40% its original volume. What is its final temperature? Homework Equations delta U = Q - W W = the integral of PdV PVgamma = constant U = 5/2nKT = 5/2nRT (diatomic, ideal gas) The Attempt at a Solution...- uchicago2012
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
J
What is a first order process in particle physics
I was given the following question Which of the reactions on the right are allowed by first-order processes? For those which are not allowed, state one conservation law which is violated. What is a first order process in particle physics? i.e what does first order refer to? What would be...- j-lee00
- Thread
- Replies: 7
- Forum: High Energy, Nuclear, Particle Physics
-
L
What is a process corner and why does it impact semiconductor speed and quality?
What exactly is a process corner. Is it actually the corner on the silicon wafer? Why does speed vary in process corners? Is the doping not uniform?- likephysics
- Thread
-
- Tags
- Process Semiconductor
- Replies: 5
- Forum: Electrical Engineering
-
C
Thermodynamics - Charging Process
Homework Statement A vertical piston-cylinder device initially contains 0.2m^3 of air at 20C. The mass of the piston is such that it maintains a constant pressure of 300kPa inside. Now a valve connected to the cylinder is opened, and air is allowed to escape until the volume inside the...- cowpuppy
- Thread
- Replies: 1
- Forum: Engineering and Comp Sci Homework Help
-
P
Specific heat in izobaric process
Homework Statement how can I calculate specific heat at constant pressure from mass specific heat and mole mass?- player1_1_1
- Thread
-
- Tags
- Heat Process Specific Specific heat
- Replies: 4
- Forum: Introductory Physics Homework Help
-
D
What is the final pressure of an adiabatically compressed gas in a container?
Homework Statement a container with air (M = 29 g/mol, treated as an ideal gas). In the container, we have 2.0 L of the gas with p1 = 1.0 atm at T1 = – 50C. The gas is compressed adiabatically to a final volume of 0.5 L. What is its final pressure? Homework Equations I am really not...- de3tz
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
M
Isobaric Process finding G using (deltaG-deltaGo)
Can anyone help me with understanding what is going on at page 11 of these notes? http://www.chem.utoronto.ca/coursenotes/CHM223/Section%205B%20Fall%202010.pdf Do you get why there is 2 delta Gs? I am really confused with what he was trying to do there? ΔG(37oC) – ΔG(25oC) ≈ – (T – 298)...- mikece
- Thread
- Replies: 1
- Forum: Classical Physics
-
Z
Entropy of Irreversible Process for 2nd Law Proof
My textbook tries to prove that for Entropy of Irreversible Process > 0 by using the example of irrev expasion into a vacuum which doesn't make sense to me. If work is = 0 then no heat is exchanged between the sys and surrounding so qsurr = 0 s(uni) = s(sys) + 0 since S(sys)=qirr/t and no...- zmike
- Thread
- Replies: 4
- Forum: Thermodynamics
-
T
Thermodynamics problem - adiabatic process
Homework Statement One cylinder in the diesel engine of a truck has an initial volume of 600 cm^3. Air is admitted to the cylinder at 35 C and a pressure of 1.0 atm. The piston rod then does 500 J of work to rapidly compress the air. What is the final temperature and volume? I found the final...- Twoism
- Thread
- Replies: 5
- Forum: Introductory Physics Homework Help
-
T
Assistance with gambit meshing process for 3d pump impeller
Hello, As part of my final year project I am to run a CFD analysis of a pump impeller. I have been provided with the impeller and diffuser CAD models and successfully imported these into gambit. However, after completing a number of the gambit tutorials I still have no experience in meshing...- themj_1
- Thread
-
- Tags
- 3d Assistance Meshing Process Pump
- Replies: 1
- Forum: Mechanical Engineering
-
R
Which chemical process causes RNA or DNA replication?
hi, DNA and RNA seem to be very peculiar molecular structures that are able to replicate themselves, and they are unique regarding this point, right?. But which chemical "magical" phenomena is responsible for this ability to reproduce?- relativityfan
- Thread
-
- Tags
- Chemical Dna Process Replication Rna
- Replies: 8
- Forum: Biology and Medical
-
M
Troubleshooting Cracks in NiTiHf Alloy Produced Through VIM Process
Hi guys I have produced an ingot of NiTiHf alloy through Vacuum Induction Melting (VIM) process and by using pure materials of Ni, Ti and Hf. Casting was done in a Y2O3-coated steel mold, and the crucible was made of graphite. The problem is that, when I homogenize the ingot at 1000 oC and...- mah65
- Thread
- Replies: 2
- Forum: Materials and Chemical Engineering
-
E
Understanding L2M2M3 Auger Process
Hi there Can someone teach me how a L2M2M3 Auger process looks like? I appreciate every kind of help.- eintagsfliege
- Thread
-
- Tags
- Process
- Replies: 4
- Forum: Atomic and Condensed Matter
-
U
Process flow diagram: pressure and velocity changes with temperature
Pressure and velocity changes with temperature in open flow tube Hello everyone, I have a process in which a gas goes through a heater. I want to calculate the physical properties of the stream coming out of the heater. Here is a description of the streams: Input to the heater: molar...- uby
- Thread
- Replies: 4
- Forum: Materials and Chemical Engineering
-
M
How does the heating process work and can it be reversed?
Hi guys, For today's lunchtime challenge... If I put 1kw of electrical energy into a heating element and get X amount of heat out, why can't I put ~X amount of heat into the element and get ~1kw of electricity out? ( I accept less than 100% reverse process ability) what's the mechanism...- motorman
- Thread
- Replies: 4
- Forum: Thermodynamics
-
C
Understanding Gear Profiles: Specification Process
pls i need help on this one... i would like to know how gear profiles are specified- chinny1388
- Thread
- Replies: 1
- Forum: Mechanical Engineering