- #1
samy4408
- 62
- 9
- TL;DR Summary
- the saturation of Hb related to its partial pressure
Hello, I just learned about the binding capacity of Hemoglobin (Hb) and that it is proportional to the partial pressure of O2 in the blood here is the curve :
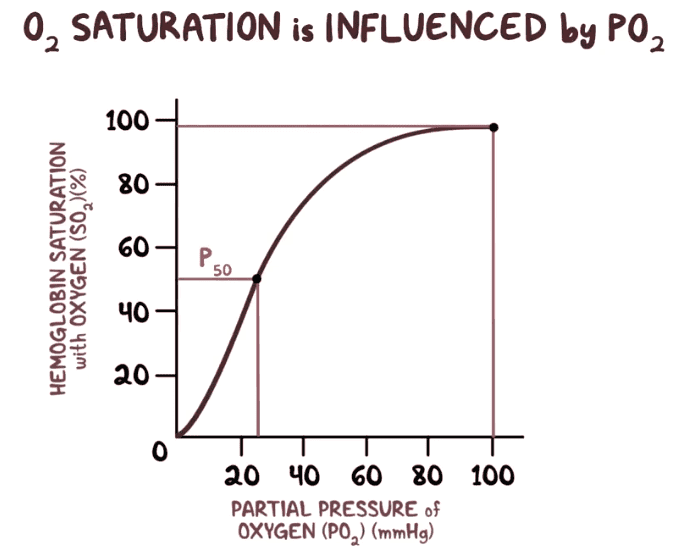
it does have a sigmoidal shape, but here is the problem: in the lecture, it's said that Hb has an increasing affinity to O2 the more O2 is bound to it, isn't it the opposite since we have to put way more partial pressure to go from 80% to 100% saturation than to go from 0% to 20%.
hoping for a reply .thanks.
it does have a sigmoidal shape, but here is the problem: in the lecture, it's said that Hb has an increasing affinity to O2 the more O2 is bound to it, isn't it the opposite since we have to put way more partial pressure to go from 80% to 100% saturation than to go from 0% to 20%.
hoping for a reply .thanks.
Last edited: