- #1
S Aditya
- 2
- 0
I was going through the chapter Chemical Bonding in one of the books and found something about orbital dipole due to lone pairs.
In each diagram the orbital dipole due to lone pair was directed from the central atom to the end of the hybridized orbital (lone pair).
Why is that so?
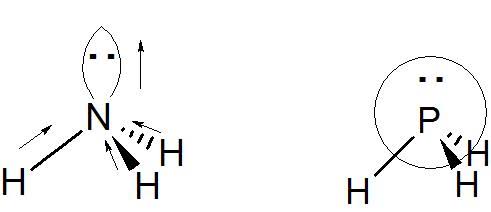
In each diagram the orbital dipole due to lone pair was directed from the central atom to the end of the hybridized orbital (lone pair).
Why is that so?