- #1
Happiness
- 679
- 30
The probability distribution of the position of the electron of a hydrogen atom is related to the following polar plots
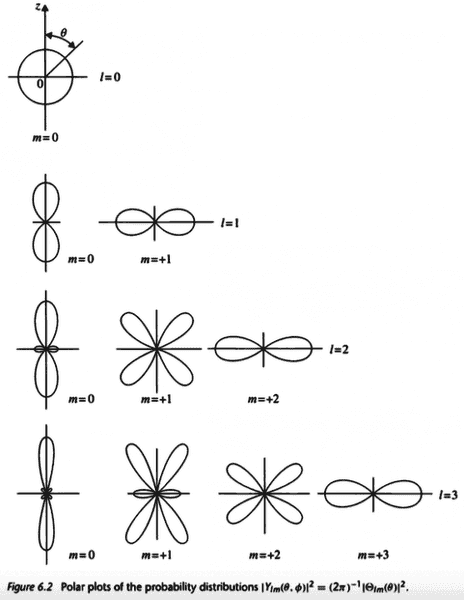
Suppose the electron is excited from the ##1s## orbital to the ##2p_x## orbital. Does it make sense to talk about the ##2p_x## orbital having a dumbbell shape pointing in the ##x## direction since the ##z## axis can be pointing in any direction? Shouldn't all orbitals be spherical when we consider there is an equal probability for the ##z## axis to point in any direction?
Suppose the electron is excited from the ##1s## orbital to the ##2p_x## orbital. Does it make sense to talk about the ##2p_x## orbital having a dumbbell shape pointing in the ##x## direction since the ##z## axis can be pointing in any direction? Shouldn't all orbitals be spherical when we consider there is an equal probability for the ##z## axis to point in any direction?