- #1
puddleduck
- 12
- 2
My professor drew the following molecule on the board and asked us how many pi electrons this aromatic molecule has.
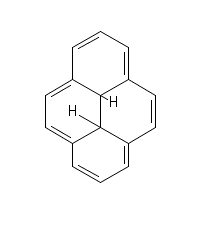
Everyone in the class said 14, as there are 7 double bonds, with two pi electrons from each bond.
He told us that there are only 10 pi electrons in this molecule, refused to explain why there were 10 instead of 14, and berated us for not understanding why. My study group and I have spent the last week pouring over textbooks and websites and none of us, nor anyone in the class, can understand why there are only 10 pi electrons. So I'm turning to y'all.
What are we missing here? Can anyone give me a clue?
(And sorry for the weird drawing - the two bonds in the center of the molecule to the two hydrogens should be the same length but I was having trouble with the online drawing tool. He also didn't specify the stereochemistry of anything, so I'm not sure if they are cis or trans to each other. Would that affect it?)
Everyone in the class said 14, as there are 7 double bonds, with two pi electrons from each bond.
He told us that there are only 10 pi electrons in this molecule, refused to explain why there were 10 instead of 14, and berated us for not understanding why. My study group and I have spent the last week pouring over textbooks and websites and none of us, nor anyone in the class, can understand why there are only 10 pi electrons. So I'm turning to y'all.
What are we missing here? Can anyone give me a clue?
(And sorry for the weird drawing - the two bonds in the center of the molecule to the two hydrogens should be the same length but I was having trouble with the online drawing tool. He also didn't specify the stereochemistry of anything, so I'm not sure if they are cis or trans to each other. Would that affect it?)

