- #1
learn.steadfast
- 59
- 2
I'm interested in the the band theory of solids for semiconductor modeling.
I haven't solved the Schrodinger equation for multiple atoms, but I'd like to know some details that experienced physicists might already know.
Several texts show how the bands develop from molecular coupling of orbitals; often the description is based on hydrogen since that makes math simpler in ignoring lots of un-necessary electrons. The orbital energies, whether filled with actual electrons or not, split. A typical diagram in undergrad courses is the following. As the atoms are brought close, bands form from distinct orbitals. The number of energy levels inside each band is proportional to the number of atoms being condensed into a solid.
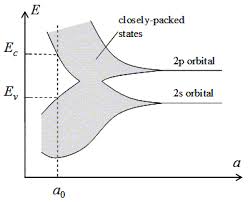
As n atoms are brought closer to each other, each orbital will split into 'n' energy levels.
My understanding is that half of the energy levels are bonding, and half are anti-bonding; at least with a hydrogen molecule that is proven to be the case. With only two levels, it's trivial that one energy level is the "bonding" state, and the other the "anti-bonding"; But I'm not sure what happens when more than two hydrogen atoms are brought together with the same spacing as the previous hypothetical molecule; Would the "top" most energy value be the same as before, or would the "anti-bonding" energy change in value, and can it be approximated from knowledge of the original anti-bonding value? eg: does splitting purely "interpolate" the space between fixed bonding and anti-bonding energies at a given atomic spacing; or does the band's height also change vs.. number of atoms in a "crystal" of an arbitrary but fixed atomic spacing?
I ask, because I think the same qualitative properties should exist in real semiconductors and would help modeling of semiconductors for electronics.
I am not able to solve Schrodinger's sufficiently to get a realistic answer for larger atoms or crystals. Normally, orbitals increase in energy with the inverse square law of their orbit number. $$ E \varpropto { R_h \over n^2 } $$ But, conventional wisdom is that the spacing of the n energy levels inside each band is equal, and I'm not completely convinced of why this should be so.
Models, like Krog & Penny, don't really deal with how bands migrate as groups up and down in energy, or how the spacing of energy levels would change vs. atomic separation. Is the energy spacing inside the band really uniform as would be predicted by simplistic models?
In the above graph, the energy levels inside the "bands" is not traced out.
How do they split in detail? at first, (tracing from right side of the graphs to left), both S and P bands seems to grow equally above and below the original energy level of their respective orbitals (which suggests symmetry, and likely linearity of splitting); but at a certain distance, the bands do opposite things. All 2p orbitals go up in energy, but the S orbitals all go down in energy.
What I would like is reasonable and simple models that give (even if crude) the statistics of how individual bands morph vs. atomic spacing.
For example, the m'th energy level inside a 3S band of n atoms; is it even crudely predictable as to where it will be relative to the band's edge or center? (linear or quadratic, etc.) Do all S orbitals go down the same amount of energy for the same change in atomic spacing, or is it inversely proportional to the square of the orbital number, etc.
For another example: It's clear from the diagram, that the 2S band gets taller vs. 'a' spacing, until a spot near a0, but then starts shrinking again. But I have no idea why, or what form of equation would qualitatively describe the S band's difference in height at any give atomic spacing 'a', it's center, or the absolute position of it's top or bottom.
Thanks.
I haven't solved the Schrodinger equation for multiple atoms, but I'd like to know some details that experienced physicists might already know.
Several texts show how the bands develop from molecular coupling of orbitals; often the description is based on hydrogen since that makes math simpler in ignoring lots of un-necessary electrons. The orbital energies, whether filled with actual electrons or not, split. A typical diagram in undergrad courses is the following. As the atoms are brought close, bands form from distinct orbitals. The number of energy levels inside each band is proportional to the number of atoms being condensed into a solid.
As n atoms are brought closer to each other, each orbital will split into 'n' energy levels.
My understanding is that half of the energy levels are bonding, and half are anti-bonding; at least with a hydrogen molecule that is proven to be the case. With only two levels, it's trivial that one energy level is the "bonding" state, and the other the "anti-bonding"; But I'm not sure what happens when more than two hydrogen atoms are brought together with the same spacing as the previous hypothetical molecule; Would the "top" most energy value be the same as before, or would the "anti-bonding" energy change in value, and can it be approximated from knowledge of the original anti-bonding value? eg: does splitting purely "interpolate" the space between fixed bonding and anti-bonding energies at a given atomic spacing; or does the band's height also change vs.. number of atoms in a "crystal" of an arbitrary but fixed atomic spacing?
I ask, because I think the same qualitative properties should exist in real semiconductors and would help modeling of semiconductors for electronics.
I am not able to solve Schrodinger's sufficiently to get a realistic answer for larger atoms or crystals. Normally, orbitals increase in energy with the inverse square law of their orbit number. $$ E \varpropto { R_h \over n^2 } $$ But, conventional wisdom is that the spacing of the n energy levels inside each band is equal, and I'm not completely convinced of why this should be so.
Models, like Krog & Penny, don't really deal with how bands migrate as groups up and down in energy, or how the spacing of energy levels would change vs. atomic separation. Is the energy spacing inside the band really uniform as would be predicted by simplistic models?
In the above graph, the energy levels inside the "bands" is not traced out.
How do they split in detail? at first, (tracing from right side of the graphs to left), both S and P bands seems to grow equally above and below the original energy level of their respective orbitals (which suggests symmetry, and likely linearity of splitting); but at a certain distance, the bands do opposite things. All 2p orbitals go up in energy, but the S orbitals all go down in energy.
What I would like is reasonable and simple models that give (even if crude) the statistics of how individual bands morph vs. atomic spacing.
For example, the m'th energy level inside a 3S band of n atoms; is it even crudely predictable as to where it will be relative to the band's edge or center? (linear or quadratic, etc.) Do all S orbitals go down the same amount of energy for the same change in atomic spacing, or is it inversely proportional to the square of the orbital number, etc.
For another example: It's clear from the diagram, that the 2S band gets taller vs. 'a' spacing, until a spot near a0, but then starts shrinking again. But I have no idea why, or what form of equation would qualitatively describe the S band's difference in height at any give atomic spacing 'a', it's center, or the absolute position of it's top or bottom.
Thanks.
Last edited: