- #1
Urmi Roy
- 753
- 1
Homework Statement
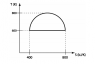
In the thermodynamic cycle as shown,
a) What is the direction that the cycle is executed-clockwise or anticlockwise?
b) What is the work done?
Homework Equations
First law of thermodynamics for a cycle
E2-E1= Q-W= Tds-Pdv=0
The Attempt at a Solution
a) I know that it should be clockwise because I read that when ∫Tds is positive, the cycle on a T-S diagram should be represented in the positive direction. However, I don't understand this completely.
b) I know it is the area inside the semicircle, but I don't know how I'm going to integrate it.