- #1
gfd43tg
Gold Member
- 950
- 50
Hello,
I am working on a process design project and was wondering if I could get some help. While this is classwork material, I thought this is too big of a problem to post in the homework section, and would be more suitable in a forum such as this one which has much less traffic. For reference, here is the project description
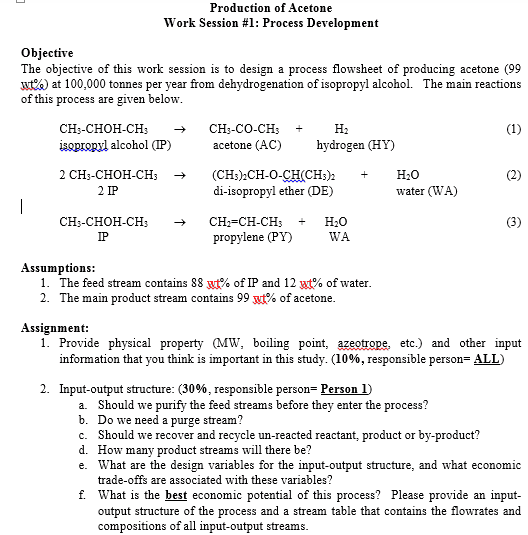

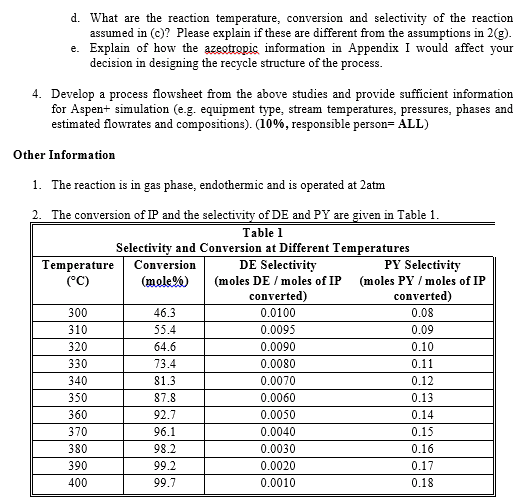

So with that said, we have 5 heuristic steps to dealing with planning a process
level 1: Batch vs. continuous
level 2: input-output structure
level 3: Recycle structure
level 4: Separation structure
level 5: Preliminary process flow sheet
1. So the production here is 100,000 tonnes/year, so it is clear this must be a continuous process (rule of thumb is > 1 million lbs/year should be continuous, we far exceed that amount)
2.
----------------------------------------------------------------------------------------------------------------------------------------------
To be continued
I am working on a process design project and was wondering if I could get some help. While this is classwork material, I thought this is too big of a problem to post in the homework section, and would be more suitable in a forum such as this one which has much less traffic. For reference, here is the project description
So with that said, we have 5 heuristic steps to dealing with planning a process
level 1: Batch vs. continuous
level 2: input-output structure
level 3: Recycle structure
level 4: Separation structure
level 5: Preliminary process flow sheet
1. So the production here is 100,000 tonnes/year, so it is clear this must be a continuous process (rule of thumb is > 1 million lbs/year should be continuous, we far exceed that amount)
2.
----------------------------------------------------------------------------------------------------------------------------------------------
To be continued


