- #1
prehisto
- 115
- 0
Hi,
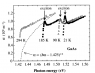
I have measured conventional absorption spectra of semiconductor - Optical density from wavelenght.
So now I want to evaluate band gap for this material.
In what kind of coordinates I how to reperesnt this spectra to see the band gap value ( where graph crosses the Energy axis) ?
*I m investigating semicondcutor
I have measured conventional absorption spectra of semiconductor - Optical density from wavelenght.
So now I want to evaluate band gap for this material.
In what kind of coordinates I how to reperesnt this spectra to see the band gap value ( where graph crosses the Energy axis) ?
*I m investigating semicondcutor
Last edited: