- #1
aname
- 8
- 0
- Homework Statement
- i'm not sure I used the numbers right
- Relevant Equations
- Determination of the dissolution enthalpy
-(7.455 so,94+8,4) Delta T
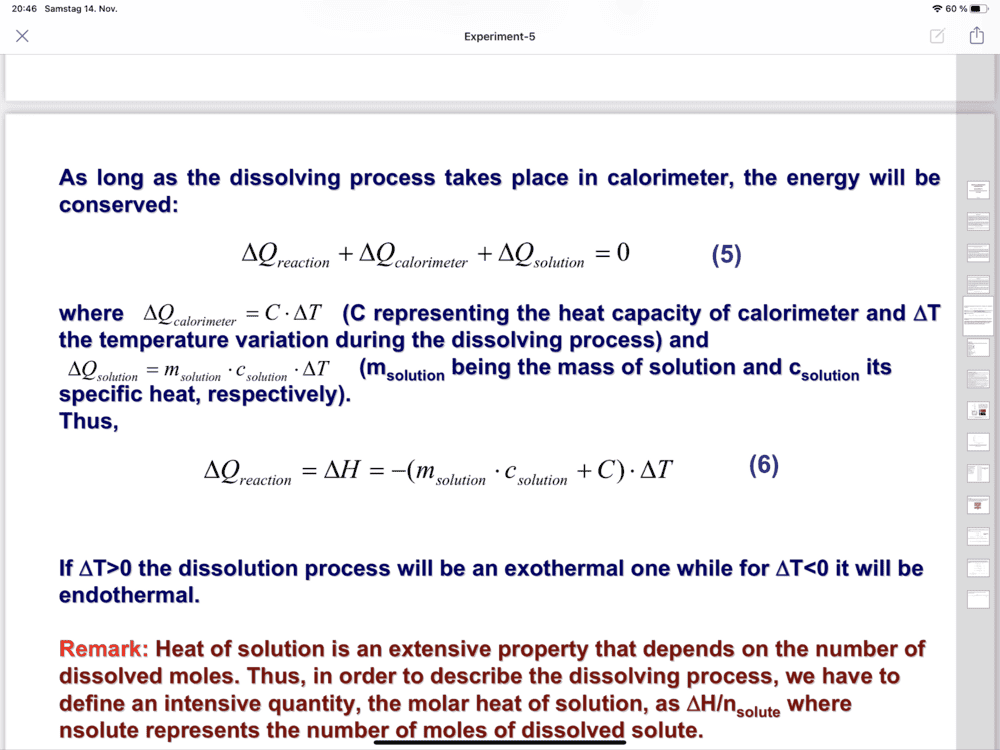
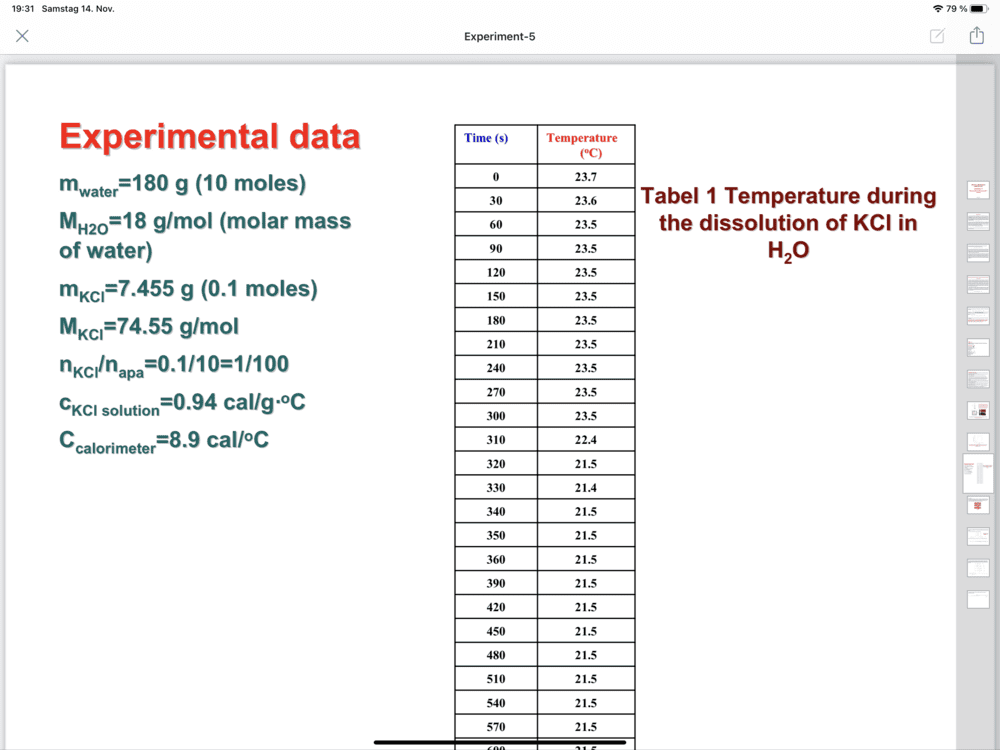
Hi,Chestermiller said:No. m of the solution is 187.5 g.
Delta H, also known as enthalpy change, is the change in energy of a system during a chemical reaction.
Delta H is calculated by taking the sum of the enthalpy of the products minus the sum of the enthalpy of the reactants.
The units for Delta H are typically in kilojoules per mole (kJ/mol).
This calculation represents the sum of the enthalpy of the products in a chemical reaction, where 7.455 represents the enthalpy of one product, 94 represents the enthalpy of another product, and 8.4 represents the enthalpy of a third product.
Delta H is used to determine whether a chemical reaction is exothermic (releases energy) or endothermic (absorbs energy). It can also be used to calculate the amount of heat released or absorbed in a reaction.