- #1
Bolter
- 262
- 31
- Homework Statement
- See below
- Relevant Equations
- pressure = rho x g x h
Having some trouble in answering this question
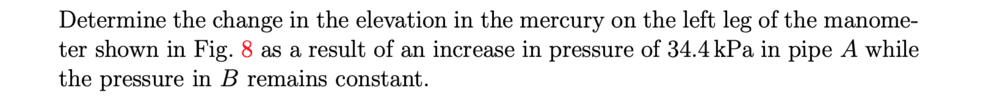
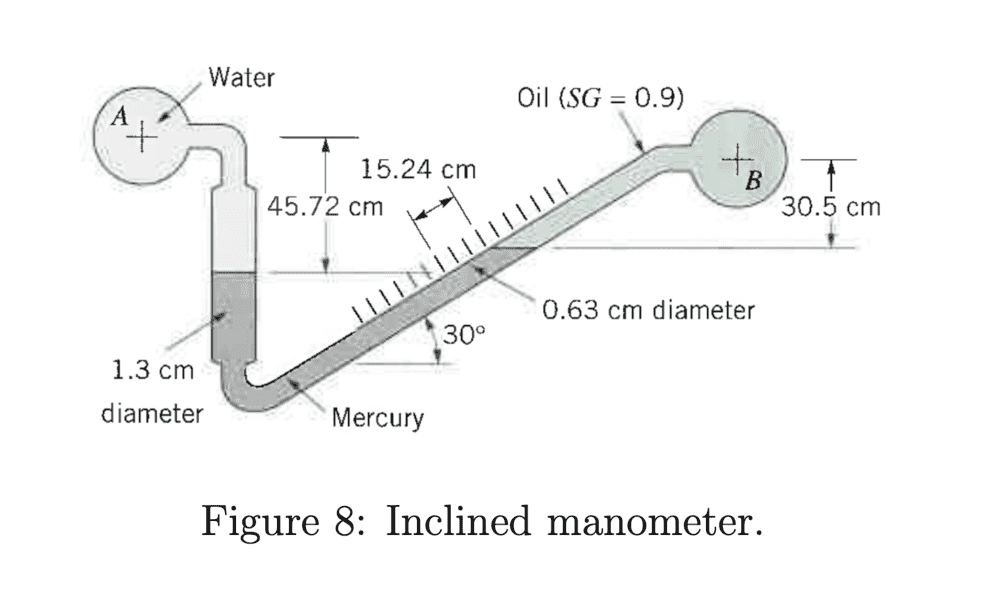
What I have done so far is calculate the initial pressure difference, then used that to find the new pressure difference when pipe's A pressure increases by 34.4 kPa
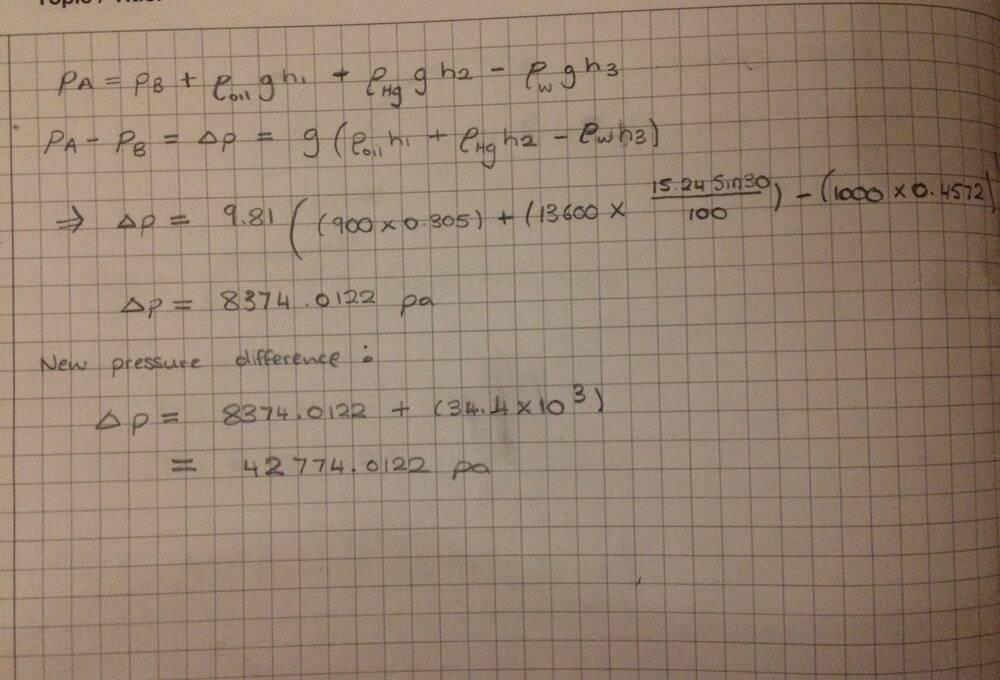
Not too sure of where to carry on from here? All I know is that if pipe A is exerting a bigger pressure then the mercury level on the left arm would decrease. But I can't seem to figure by how much this decrease would be
I would be grateful for any help. Thanks!
What I have done so far is calculate the initial pressure difference, then used that to find the new pressure difference when pipe's A pressure increases by 34.4 kPa
Not too sure of where to carry on from here? All I know is that if pipe A is exerting a bigger pressure then the mercury level on the left arm would decrease. But I can't seem to figure by how much this decrease would be
I would be grateful for any help. Thanks!