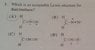- #1
runningman19
- 19
- 3
Homework Statement
Which is an acceptable Lewis structure for Diazomethane?
Homework Equations
None
The Attempt at a Solution
The answer cannot be C or D, as both of these resonance structures are incorrect. I thought the answer was A, given that nitrogen is more electronegative than carbon, which would mean that a negative formal charge is more stable on nitrogen. This is not the case, the answer is actually B.