- #1
metastable
- 514
- 53
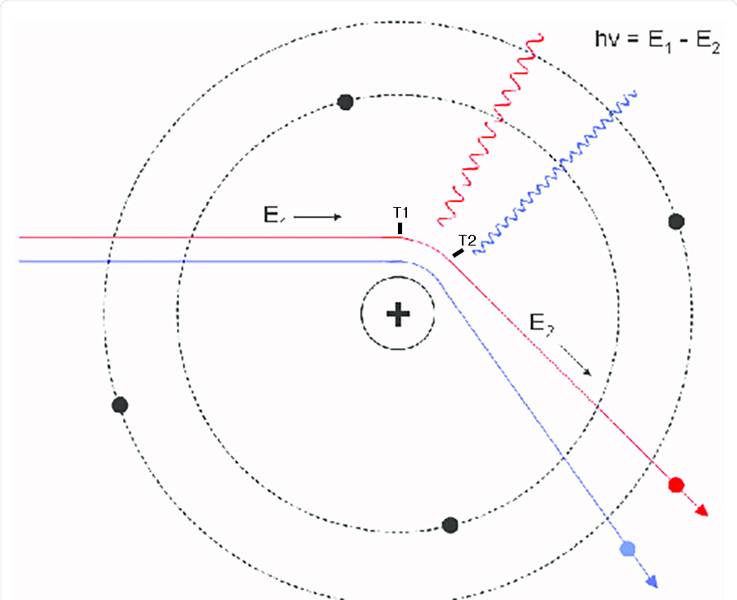
If I'm scattering electrons off of an atomic nucleus, can I infer any information about the duration of time the electron was accelerating by looking at the wavelength of the emitted bremsstrahlung radiation? I am wondering if it would be possible to derive a time interval between electron acceleration start (T1) and acceleration end (T2) by looking at the bremsstrahlung photon frequency.