- #1
Pushoam
- 962
- 51
// moved from general forum, homework template missing //
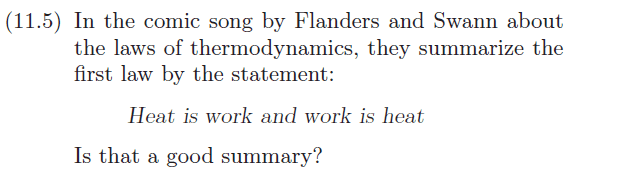
Assuming that by the word "Work", work on the system is meant.
Both heat and work is energy in transit.
I think heat is work done by constituent particles of a system on another system in contact.
Now the work done by us on a system gets transformed into the K.E. and P.E. of the system.
In thermodynamics, here it is assumed that the change in the P.E. is 0. So, work done by us is nonthing but the change in the K.E. of the system.
This change in the K.E. of the system is nothing but the change in the average K.E. of the constituent particles of the system. Now this change in the average kinetic energy of the constituent particles is nothing but the work done on the constituent particles , hence, heat.
Is this right?
Assuming that by the word "Work", work on the system is meant.
Both heat and work is energy in transit.
I think heat is work done by constituent particles of a system on another system in contact.
Now the work done by us on a system gets transformed into the K.E. and P.E. of the system.
In thermodynamics, here it is assumed that the change in the P.E. is 0. So, work done by us is nonthing but the change in the K.E. of the system.
This change in the K.E. of the system is nothing but the change in the average K.E. of the constituent particles of the system. Now this change in the average kinetic energy of the constituent particles is nothing but the work done on the constituent particles , hence, heat.
Is this right?
Last edited by a moderator: