- #1
yaro99
- 75
- 0
- Homework Statement
- Reproduce the results in Figure 4.20 (shown in post). [Exergy loss in a liquefaction system].
- Relevant Equations
- Energy Balance: dE_cv/dt = Q_cv - W_cv + SIGMA [mdot_i (h_i + V_i^2+gzi)] - [mdot_e (h_e + V_e^2+gze)]
Exergy Balance: dE/dt = (1- T_0/T_j)*Q_j - W_cv +SIGMA [ (m_i*e_fi) - (m_e*e_fe) ] - Edot)
Entropy: (Q_dot,cv/m_dot)_int,rev = int|1-2(T ds)
Q_dot,cv/m_dot = eta * (Q_dot,cv/m_dot)_int,rev
Problem, with state values, and pie chart (Fig 4.20) showing answers:
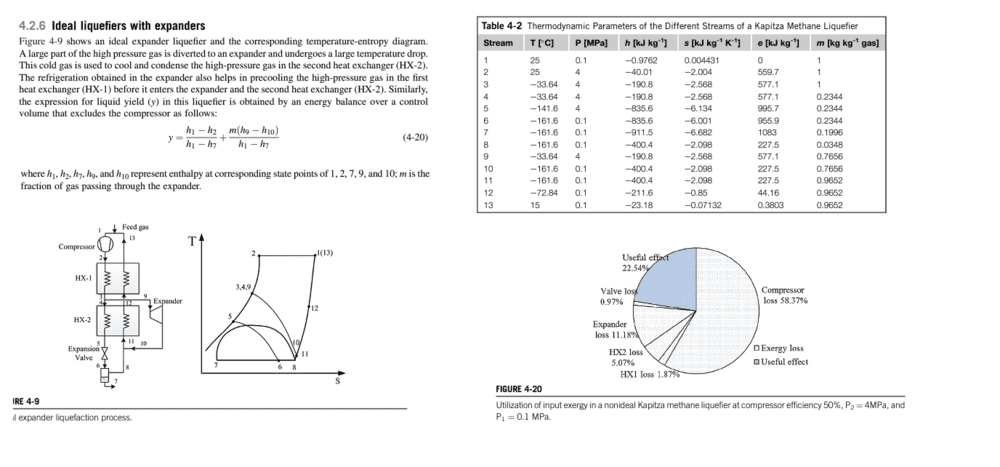
^ This shows the system in question (Kapitza Liquefaction System). Methane gas enters into the compressor (c), then goes through the first heat exchanger (HX1). Some of it (z) gets routed to the expander (exp). Afterwards, the rest goes to the second heat exchanger (HX2), then to the Expansion Valve (JT). Then, the liquefied methane is collected (state 7), and the rest is routed back, first to HX2, then HX1, before going right back to the compressor, with a certain amount being supplied between state 13 and state 1.
Using Table 4-2, here are the results I obtained:
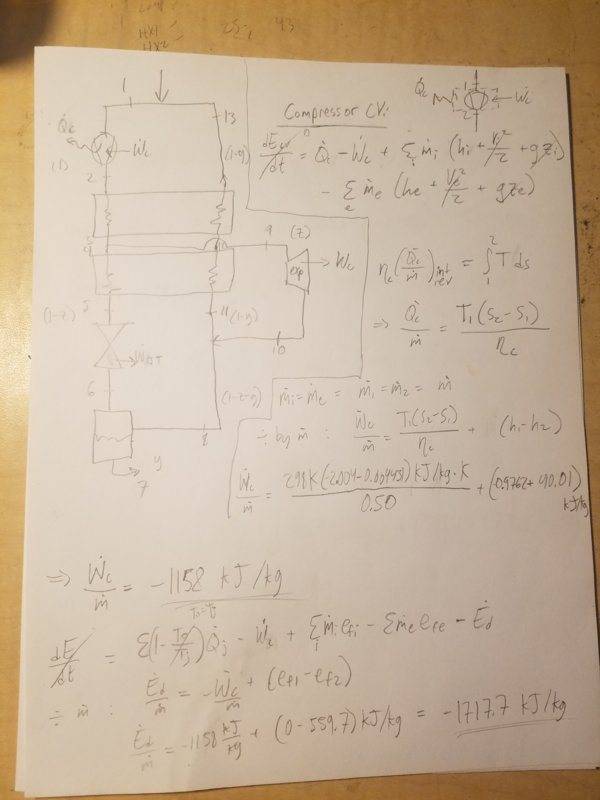
1. Compressor
2. Heat Exchanger 1
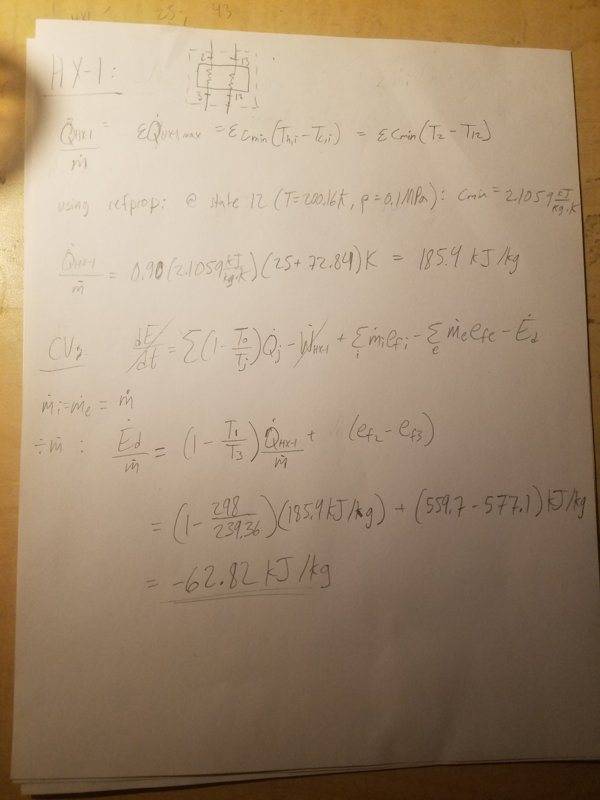
3. Heat Exchanger 2
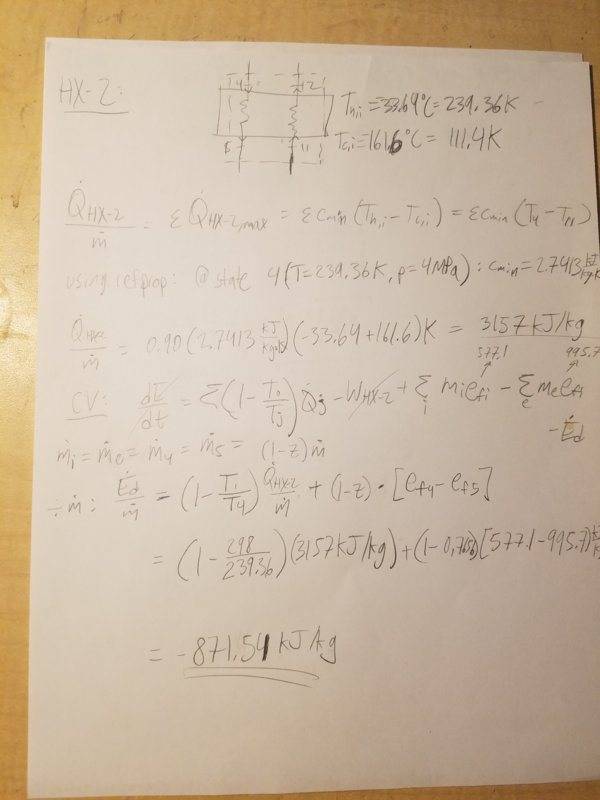
4. Expansion Valve
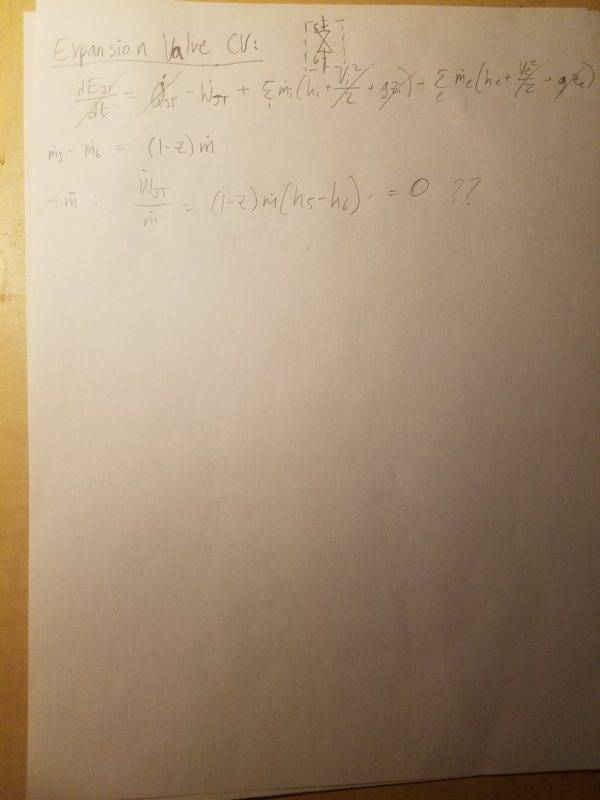
5. Expander
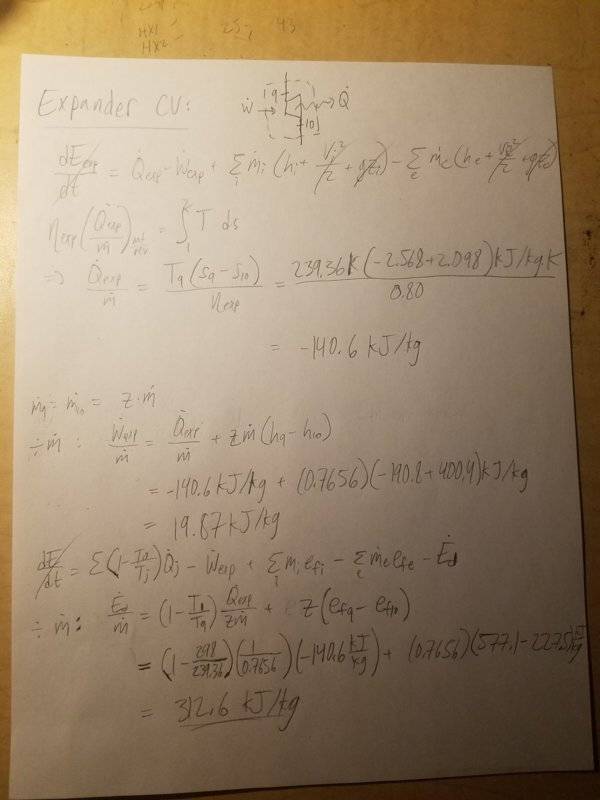
Results)
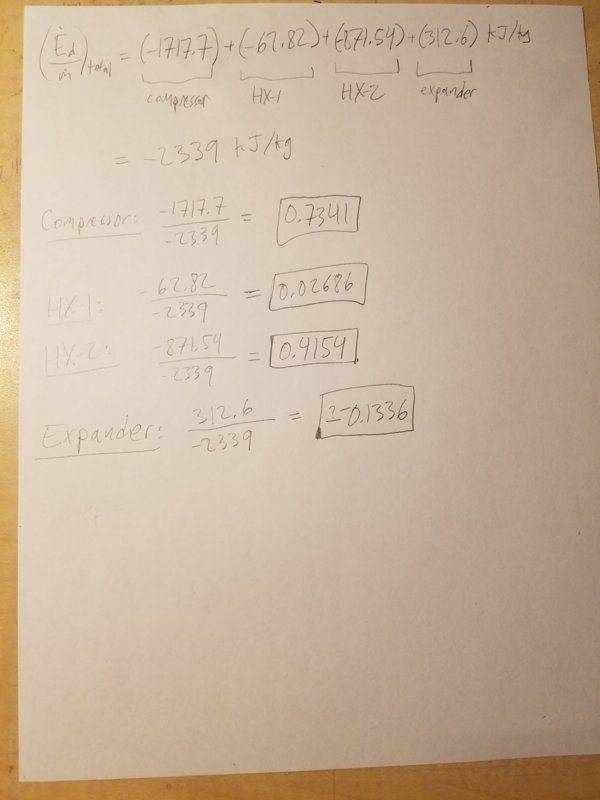
My thermodynamics is a pretty rusty, so forgive me if I'm making a simple mistake, but I just haven't been able to find sources to help me out on this one.
I was wondering about the control volumes around each unit, namely the heat exchangers, the expander, and the expansion valve (is there work, heat transfer, both, or neither?). I'm sorry if this seems obvious but hours of research online and in my textbooks have not helped too much.
There are two main things I'm confused about: 1) My expansion valve analysis ends up with zero work (as the enthalpies are the same in both states), and there's no way this can be true, and 2) My exergy analysis on the expander results in negative exergy, which is not possible.
^ This shows the system in question (Kapitza Liquefaction System). Methane gas enters into the compressor (c), then goes through the first heat exchanger (HX1). Some of it (z) gets routed to the expander (exp). Afterwards, the rest goes to the second heat exchanger (HX2), then to the Expansion Valve (JT). Then, the liquefied methane is collected (state 7), and the rest is routed back, first to HX2, then HX1, before going right back to the compressor, with a certain amount being supplied between state 13 and state 1.
Using Table 4-2, here are the results I obtained:
1. Compressor
2. Heat Exchanger 1
3. Heat Exchanger 2
4. Expansion Valve
5. Expander
Results)
My thermodynamics is a pretty rusty, so forgive me if I'm making a simple mistake, but I just haven't been able to find sources to help me out on this one.
I was wondering about the control volumes around each unit, namely the heat exchangers, the expander, and the expansion valve (is there work, heat transfer, both, or neither?). I'm sorry if this seems obvious but hours of research online and in my textbooks have not helped too much.
There are two main things I'm confused about: 1) My expansion valve analysis ends up with zero work (as the enthalpies are the same in both states), and there's no way this can be true, and 2) My exergy analysis on the expander results in negative exergy, which is not possible.
 for this playing stumm.
for this playing stumm.