- #1
Richie Smash
- 293
- 15
Homework Statement
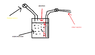
Here is a pesticide spray vessel, of total volume of 10 litres, containing 8 litres of pesticide.
Valve B is closed first,and the space above the air is filled with air at a pressure of 200KPa until the desired pressure is reached, and then valve A is then closed.
The sprayer is then used for sometime until the liquid level falls and the pressure of the air in the space above the liquid is reduced
Calculate the volume of the liquid that would still be in the tank when air pressure above the tank is 150KPa and all temperatures reamin constant.
Assume all pressure differences due to hydrostatic pressure of liquid to be negligible; i.e pressure at upper surface of liquid = pressure at bottom of the tank.
(Atmospheric pressure =100Kpa)
Homework Equations
P1V1=P2V2
The Attempt at a Solution
Hi I originally thought the solution would be to use Boyle's law, but I realized its for the volume of pesticide not the air.
And normally I would think, if the initial pressure of the air pumped was 200Kpa, would I have to add atmospheric pressure to that, but that can't be since it's in a container?
I'm very stumped as this is one of my worst areas in physics.
*high school level*