- #1
shadedvertex
- 2
- 0
If we assume the energy of particles in an ideal gas follows a Boltzmann distribution, then the energy distribution function can be defined as below:
 , where k_B is the Boltzmann constant
, where k_B is the Boltzmann constant
Since the energy of particles in an ideal gas are assumed to only consist of translational kinetic energy (as they don't interact),
 , where v is the speed of the particle
, where v is the speed of the particle
However, for a certain level of kinetic energy, there is only one speed that can be associated with it, as speeds take positive value and hence there is a one-to-one relationship between the speed of a particle and its kinetic energy.
By this reasoning, the distribution function of energy should be proportional to the distribution function of velocity. In other words, the velocity distribution function is also proportional to a Boltzmann factor, with a constant factor of proportionality.
However, the Maxwell-Boltzmann speed distribution shows the velocity distribution having the following form:
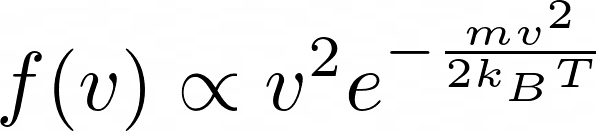
Although I can follow the derivation of the Maxwell-Boltzmann distribution, I fail to see why the line of reasoning I described in previous paragraphs is wrong. Any help would be much appreciated. Thank you!
Since the energy of particles in an ideal gas are assumed to only consist of translational kinetic energy (as they don't interact),
However, for a certain level of kinetic energy, there is only one speed that can be associated with it, as speeds take positive value and hence there is a one-to-one relationship between the speed of a particle and its kinetic energy.
By this reasoning, the distribution function of energy should be proportional to the distribution function of velocity. In other words, the velocity distribution function is also proportional to a Boltzmann factor, with a constant factor of proportionality.
However, the Maxwell-Boltzmann speed distribution shows the velocity distribution having the following form:
Although I can follow the derivation of the Maxwell-Boltzmann distribution, I fail to see why the line of reasoning I described in previous paragraphs is wrong. Any help would be much appreciated. Thank you!