- #1
Bolter
- 262
- 31
- Homework Statement
- See below
- Relevant Equations
- Q = mc(deta T)
Hi everyone
This is a quick Q but I don't understand why I got it wrong

This is what I have done
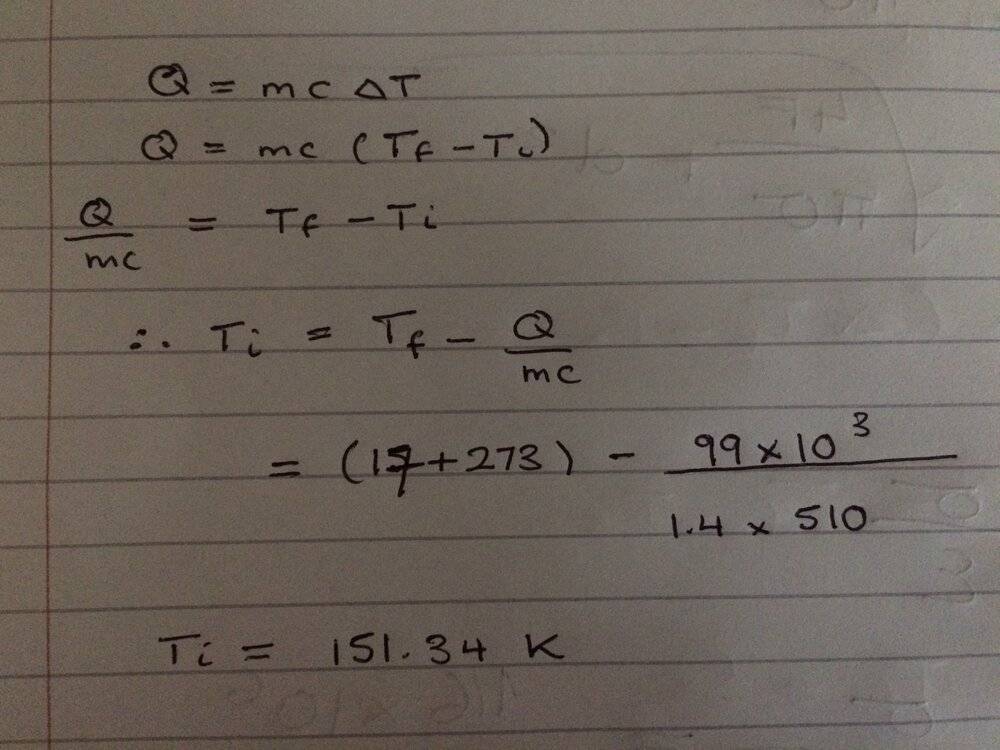
Could anyone let me know where I went wrong? Thanks for any help!
This is a quick Q but I don't understand why I got it wrong
This is what I have done
Could anyone let me know where I went wrong? Thanks for any help!