- #1
Kalkunipraed
- 4
- 0
Short version:
If I have an insulated container(box) of some specific size. The inside temperature is temperature X. Ambient temperature is Y. Also X < Y. The box starts warming up. I log the inside temperature over time with data points.
How can I determine one or more of the following based on the data points:
A) The amount of energy gained in Watts (for some specific temperature difference)
B) Thermal resistance value for the box
C) Thermal conduction value for the box
Long version:
I am a homebrewer and built an insulated box for fermentation.
The container has walls of 5cm polystyrene (EPS), 2cm polystyrene(XPS) and 6mm plywood.
The container outer dimensions are height: 72cm(23.3inches), width and length 60cm(23.6 inches).
Inner height: 56cm(22 inches), width and depth 45cm(17.7 inches)
I took it outside where it was about 7C(44.6F) and left it there to cool it down. Then put the lid on and the temperature controller thermometer in and brought it inside where the ambient temperature was 22-23C (71.7-73.4F). I took notes how the temperature changed over time inside the box. Time units are in minutes. I didn't wait until it got to ambient, but it got rather close.
Sample data points:
0 - 7.9 C
30 - 10 C
59 - 12.9 C
100 - 16.0 C
or
0 - 46.2 F
30 - 50 F
59 - 55.2 F
100 - 60.8 F
The graph with full collected data and Celsius values:
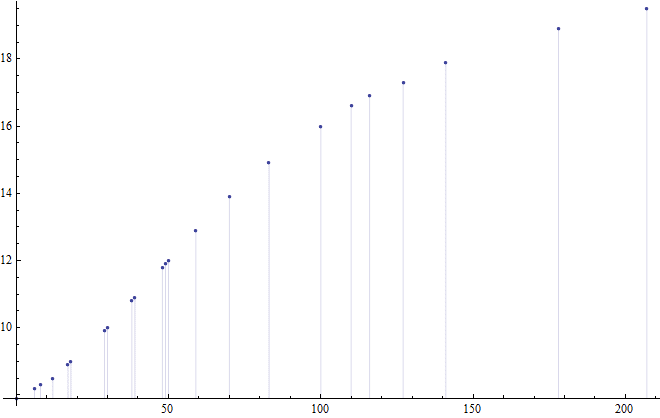
How can I determine the amount of Watts seeping into my box for any temperature difference? The answer I am trying to get is something like: for a temperature difference of 7C, the box receives 12W/h. Or anything that gives me more-or-less accurate wattage in any form.
This would help me determine how much cooling I need for the container.
I looked around the web for several hours without finding a solution. Or some solutions that I tried suggested only ~1-2W/h, which is way too low. My very rough calculations based on the insulation type suggest it should be at east 11-12W/h. I do not have the incorrect equations at hand because I am at work.
So I would be very glad if someone could help me with this.
Thank you in advance!
If I have an insulated container(box) of some specific size. The inside temperature is temperature X. Ambient temperature is Y. Also X < Y. The box starts warming up. I log the inside temperature over time with data points.
How can I determine one or more of the following based on the data points:
A) The amount of energy gained in Watts (for some specific temperature difference)
B) Thermal resistance value for the box
C) Thermal conduction value for the box
Long version:
I am a homebrewer and built an insulated box for fermentation.
The container has walls of 5cm polystyrene (EPS), 2cm polystyrene(XPS) and 6mm plywood.
The container outer dimensions are height: 72cm(23.3inches), width and length 60cm(23.6 inches).
Inner height: 56cm(22 inches), width and depth 45cm(17.7 inches)
I took it outside where it was about 7C(44.6F) and left it there to cool it down. Then put the lid on and the temperature controller thermometer in and brought it inside where the ambient temperature was 22-23C (71.7-73.4F). I took notes how the temperature changed over time inside the box. Time units are in minutes. I didn't wait until it got to ambient, but it got rather close.
Sample data points:
0 - 7.9 C
30 - 10 C
59 - 12.9 C
100 - 16.0 C
or
0 - 46.2 F
30 - 50 F
59 - 55.2 F
100 - 60.8 F
The graph with full collected data and Celsius values:
How can I determine the amount of Watts seeping into my box for any temperature difference? The answer I am trying to get is something like: for a temperature difference of 7C, the box receives 12W/h. Or anything that gives me more-or-less accurate wattage in any form.
This would help me determine how much cooling I need for the container.
I looked around the web for several hours without finding a solution. Or some solutions that I tried suggested only ~1-2W/h, which is way too low. My very rough calculations based on the insulation type suggest it should be at east 11-12W/h. I do not have the incorrect equations at hand because I am at work.
So I would be very glad if someone could help me with this.
Thank you in advance!
Last edited: